UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of May 2023
Commission File Number: 001-38281
ERYTECH Pharma S.A.
(Translation of registrant’s name into English)
60 Avenue Rockefeller
69008 Lyon France
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F:
☒ Form 20-F ☐ Form 40-F
INCORPORATION BY REFERENCE
This Report on Form 6-K and Exhibits 99.1 and 99.2 to this Report on Form 6-K shall be deemed to be incorporated by reference into the registration statements on Form F-3 (File Nos. 333-248953 and 333-259690) and registration statements on Form S-8 (File Nos. 333-222673, 333-232670, 333-239429, 333-255900 and 333-265927), of ERYTECH Pharma S.A. (the “Company”) (including any prospectuses forming a part of such registration statements) and to be a part thereof from the date on which this report is filed, to the extent not superseded by documents or reports subsequently filed or furnished.
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
On May 23, 2023, ERYTECH Pharma S.A. filed a document d’exemption (the “exemption document”) with the Autorité des marchés financiers (AMF) in France in connection with its previously announced business combination with PHERECYDES Pharma S.A. (“PHERECYDES”). An English translation version of the exemption document is attached as Exhibit 99.1 to this Report on Form 6-K and incorporated herein by reference.
The exemption document incorporates by reference, among others, PHERECYDES’ 2022 annual financial report published on April 27, 2023 (the “PHERECYDES 2022 Annual Financial Report”), including report of the Board of Directors of PHERECYDES on corporate governance included therein. An English translation version of the PHERECYDES 2022 Annual Financial Report is attached as Exhibit 99.2 to this Report on Form 6-K and incorporated herein by reference.
EXHIBIT INDEX
| Exhibit |
Description | |
| 99.1 | Document d’exemption (English translation). | |
| 99.2 | PHERECYDES 2022 Annual Financial Report (English translation). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ERYTECH Pharma S.A. | ||||||
| Date: May 24, 2023 | By: | /s/ Eric Soyer | ||||
| Name Eric Soyer | ||||||
| Title: Chief Financial Officer and Chief Operating Officer | ||||||
Exhibit 99.1
MERGER-ABSORPTION OF

BY

EXEMPTION DOCUMENT PREPARED IN CONNECTION WITH THE MERGER BY ABSORPTION OF PHERECYDES PHARMA BY ERYTECH PHARMA
This exemption document (the “Exemption Document”) is available free of charge at the registered office of Erytech Pharma (60 Avenue Rockefeller, 69008 Lyon, France) and on its website (http://www.erytech.com/).
The Exemption Document is also available in electronic format on the AMF’s website (https://www.amf-france.org/).
The Exemption Document incorporates by reference:
| • | with respect to ERYTECH Pharma (“Erytech”): the 2022 universal registration document filed with the French financial markets authority (Autorité des marchés financiers) (the “AMF”) on March 28, 2023 under number D.23-0172 (the “Erytech 2022 Universal Registration Document”); |
| • | with respect to Pherecydes Pharma: the 2022 annual financial report published on April 27, 2023 (the “Pherecydes 2022 Annual Financial Report”), the Pherecydes board of directors’ report on corporate governance included in the Pherecydes 2022 Annual Financial Report (the “Pherecydes 2022 Corporate Governance Report”).] |
In accordance with point 1.5 of Annex I of the Delegated Regulation n°2021-528, it is stated that:
| • | the Exemption Document does not constitute a prospectus within the meaning of Regulation (EU) 2017/1129; |
| • | the Exemption Document has not been subject to the scrutiny and approval by the relevant competent authority in accordance with Article 20 of Regulation (EU) 2017/1129. |
1
Definitions:
In this Exemption Document, and unless otherwise indicated:
| “ADS” | means the “American Depositary Shares” admitted to trading on the Nasdaq Capital Market. | |
| “Merger” | means the merger by absorption of Pherecydes into Erytech as described in Section 3 of the Exemption Document. | |
| “Erytech Group” | means the group of companies consisting of Erytech and its subsidiary, ERYTECH Pharma, Inc., whose registered office is located at PO Box 507 Lunenburg, MA 02462, United-States of America. | |
| “Nasdaq” | means The Nasdaq Securities Market LLC. | |
| “Memorandum of Understanding” | means the memorandum of understanding entered into on February 15, 2023 by and between Erytech and Pherecydes by which Erytech and Pherecydes have agreed on the terms of the negotiations relating to the Merger. | |
| “Absorbing Company” or “Erytech” | means ERYTECH Pharma, a public limited liability company (société anonyme), having its registered office at 60, avenue Rockefeller, 69008 Lyon, registered in the Lyon Trade and Companies Register under number 479 560 013. | |
| “Absorbed Company” or “Pherecydes” | means Pherecydes Pharma, a public limited liability company (société anonyme), having its registered office at 22, boulevard Benoni Goullin, 44200 Nantes, registered in the Nantes Trade and Companies Register under number 493 252 266. | |
| “Contribution Agreement” | means the contribution agreement entered into by and between Erytech Pharma and FCPI OUEST VENTURES III, AURIGA IV BIOSEEDS and the POOL GUY RIGAUD (as set forth in Annex 1 of the Merger Agreement) on May 5, 2023. | |
| “Merger Agreement” | means the merger agreement entered into by and between Erytech and Pherecydes on May 15, 2023. | |
2
TABLE OF CONTENTS
| 1. |
PERSONS RESPONSIBLE FOR DRAWING UP THE EXEMPTION DOCUMENT, THIRD PARTY INFORMATION AND EXPERT REPORTS | 7 | ||||
| 1.1 |
IDENTIFICATION OF PERSONS RESPONSIBLE FOR DRAWING UP THE EXEMPTION DOCUMENT | 7 | ||||
| 1.1.1 |
FOR ERYTECH, ABSORBING COMPANY | 7 | ||||
| 1.1.2 |
FOR PHERECYDES, ABSORBED COMPANY | 7 | ||||
| 1.2 |
RESPONSIBILITY STATEMENT | 7 | ||||
| 1.2.1 |
FOR ERYTECH, ABSORBING COMPANY | 7 | ||||
| 1.2.2 |
FOR PHERECYDES, ABSORBED COMPANY | 7 | ||||
| 1.3 |
EXPERT’S STATEMENT OR REPORT | 7 | ||||
| 1.4 |
INFORMATION SOURCED BY A THIRD PARTY | 8 | ||||
| 2. |
INFORMATION ON THE ISSUER AND ON THE ABSORBED COMPANY | 8 | ||||
| 2.1 |
INFORMATION ON ERYTECH, THE ABSORBING COMPANY | 8 | ||||
| 2.1.1 |
GENERAL INFORMATION | 8 | ||||
| 2.1.2 |
BUSINESS OVERVIEW | 9 | ||||
| 2.1.3 |
INVESTMENTS | 10 | ||||
| 2.1.4 |
CORPORATE GOVERNANCE | 10 | ||||
| 2.1.5 |
FINANCIAL INFORMATION | 14 | ||||
| 2.1.6 |
LEGAL AND ARBITRATION PROCEEDINGS | 14 | ||||
| 2.1.7 |
SUMMARY OF INFORMATION DISCLOSED UNDER REGULATION (EU) N° 596/2014 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL | 15 | ||||
| 2.2 |
INFORMATION ON PHERECYDES, THE ABSORBED COMPANY | 18 | ||||
3
| 2.2.1 |
GENERAL INFORMATION | 18 | ||||
| 2.2.2 |
BUSINESS OVERVIEW | 18 | ||||
| 2.2.3 |
INVESTMENTS | 21 | ||||
| 2.2.4 |
CORPORATE GOVERNANCE | 22 | ||||
| 2.2.5 |
FINANCIAL INFORMATION | 23 | ||||
| 2.2.6 |
LEGAL AND ARBITRATION PROCEEDINGS | 24 | ||||
| 2.2.7 |
SUMMARY OF INFORMATION DISCLOSED UNDER REGULATION (EU) N° 596/2014 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL | 24 | ||||
| 3. |
DESCRIPTION OF THE MERGER | 27 | ||||
| 3.1 |
PURPOSE AND OBJECTIVES OF THE MERGER |
27 | ||||
| 3.1.1 |
PURPOSE OF THE MERGER FOR THE ABSORBING COMPANY AND ITS SHAREHOLDERS | 27 | ||||
| 3.1.2 |
PURPOSE OF THE MERGER FOR THE ABSORBED COMPANY AND ITS SHAREHOLDERS | 28 | ||||
| 3.1.3 |
DESCRIPTION OF THE ANTICIPATED BENEFITS RESULTING FROM THE MERGER | 28 | ||||
| 3.2 |
CONDITIONS OF THE MERGER |
29 | ||||
| 3.2.1 |
LEGAL ASPECTS OF THE MERGER | 30 | ||||
| 3.2.2 |
MERGER CONTROL | 34 | ||||
| 3.2.3 |
MERGER CONSIDERATION | 35 | ||||
| 3.2.3.7 |
BREAK-UP FEES AND PENALTIES | 40 | ||||
| 3.2.4 |
NOTIFICATIONS AND REQUESTS FOR AUTHORIZATION | 40 | ||||
| 3.2.5 |
INFORMATION ON THE FINANCING STRUCTURE OF THE MERGER | 40 | ||||
| 3.2.6 |
MERGER TIMETABLE | 40 | ||||
| 3.3 |
RISK FACTORS RELATED TO THE MERGER |
41 | ||||
| 3.4 |
CONFLICTS OF INTERESTS |
43 | ||||
| 3.5 |
MERGER CONSIDERATION |
43 | ||||
| 4. |
EQUITY SECURITIES ADMITTED TO TRADING ON A REGULATED MARKET FOR THE PURPOSE OF THE MERGER | 44 | ||||
| 4.1 |
RISK FACTORS RELATED TO EQUITY SECURITIES |
44 | ||||
| 4.2 |
NET WORKING CAPITAL STATEMENT |
45 | ||||
4
| 4.3 |
INFORMATION CONCERNING THE EQUITY SECURITIES TO BE ADMITTED TO TRADING |
45 | ||||
| 4.3.1 |
NATURE, CLASS, AMOUNT AND CURRENCY OF ISSUE OF SECURITIES ADMITTED TO TRADING | 45 | ||||
| 4.3.2 |
RESOLUTIONS, AUTHORIZATIONS AND APPROVALS PURSUANT TO WHICH THE SECURITIES WILL BE CREATED AND/OR ISSUED | 45 | ||||
| 4.3.3 |
RESTRICTIONS ON THE FREE NEGOTIABILITY OF SECURITIES | 46 | ||||
| 4.3.4 |
TAKEOVER BIDS LAUNCHED BY THIRD PARTIES IN RESPECT OF THE ABSORBING COMPANY’S SECURITIES WHICH HAVE OCCURRED DURING THE LAST AND CURRENT FINANCIAL YEARS | 46 | ||||
| 4.4 |
ADMISSION TO TRADING AND DEALING ARRANGEMENTS |
46 | ||||
| 4.4.1 |
ADMISSION TO TRADING | 46 | ||||
| 4.4.2 |
LIQUIDITY COMMITMENT, PLACEMENT AND UNDERWRITING | 46 | ||||
| 4.4.3 |
LOCK-UP AGREEMENT - ABSTENTION AND/OR RETENTION UNDERTAKING | 46 | ||||
| 4.5 |
DILUTION |
46 | ||||
| 4.5.1 |
STRUCTURE CHART | 48 | ||||
| 4.6 |
COUNSELORS |
48 | ||||
| 5. |
IMPACT OF THE MERGER ON THE ISSUER | 48 | ||||
| 5.1 |
STRATEGY AND OBJECTIVES |
48 | ||||
| 5.2 |
MATERIAL CONTRACTS |
48 | ||||
| 5.3 |
DISINVESTMENT |
49 | ||||
| 5.4 |
CORPORATE GOVERNANCE |
49 | ||||
| 5.5 |
PARTICIPATION |
53 | ||||
| 5.5.1 |
SHAREHOLDING | 53 | ||||
| 5.6 |
PRO FORMA FINANCIAL INFORMATION |
54 | ||||
| 5.7 |
REPORT OF THE STATUTORY AUDITORS OF ERYTECH ON THE PRO FORMA FINANCIAL INFORMATION |
73 | ||||
| 6. |
AVAILABLE DOCUMENTS | 75 | ||||
5
| ANNEX 1. |
MERGER AGREEMENT |
77 | ||||
| ANNEX 2. |
REPORTS OF THE MERGER AUDITOR |
124 | ||||
| ANNEX 3. |
TEXT OF THE RESOLUTIONS OF THE GENERAL MEETINGS OF THE SHAREHOLDERS OF ERYTECH AND PHERECYDES |
104 | ||||
| ANNEX 4. |
EXTRACTS FROM THE RESOLUTIONS OF THE BOARDS OF DIRECTORS OF ERYTECH AND PHERECYDES AUTHORIZING THE MERGER PROJECT |
130 | ||||
| ANNEX 5. |
CONCORDANCE TABLE |
139 | ||||
6
| 1. | PERSONS RESPONSIBLE FOR THE EXEMPTION DOCUMENT, THIRD PARTY INFORMATION AND EXPERT REPORTS |
| 1.1 | IDENTIFICATION OF PERSONS RESPONSIBLE FOR THE EXEMPTION DOCUMENT |
| 1.1.1 | For Erytech, Absorbing Company |
Mr. Gil Beyen, Chief Executive Officer of Erytech
Persons responsible for the financial information:
Mr. Gil Beyen, Chief Executive Officer and Mr. Eric Soyer, Deputy Chief Executive Officer, Chief Financial Officer and Chief Operating Officer
Phone number: +33 4 78 74 44 38
Fax: +33 4 78 75 56 29
e-mail: investors@erytech.com
| 1.1.2 | For Pherecydes, Absorbed Company |
Mr. Thibaut du Fayet, Chief Executive Officer of Pherecydes
e-mail : investors@pherecydes-pharma.com
| 1.2 | RESPONSIBILITY STATEMENT |
| 1.2.1 | For Erytech, Absorbing Company |
“I certify that the information contained in the Exemption Document relating to Erytech Pharma is, to the best of my knowledge, in accordance with the facts and contains no omission likely to affect its import.”
Mr. Gil Beyen
Chief Executive Officer
| 1.2.2 | For Pherecydes, Absorbed Company |
“I certify that the information contained in the Exemption Document relating to Pherecydes Pharma is, to the best of my knowledge, in accordance with the facts and contains no omission likely to affect its import.”
Mr. Thibaut du Fayet
Chief Executive Officer
| 1.3 | EXPERT’S STATEMENT OR REPORT |
Ruling on the joint request of Erytech and Pherecydes, the President of the Commercial Court of Lyon has, by order of February 28, 2023, appointed Finexsi as merger auditor (commissaire à la fusion), a French public limited liability company (société anonyme) whose registered office is located at 14 rue de Bassano, 75116 Paris, France, represented by Mr. Christophe Lambert, whose mission, in accordance
7
with Article L. 236-10 of the French Commercial Code (Code de commerce), is to (i) verify that the relative values attributed to the shares of the companies participating in the Merger are relevant and that the exchange ratio is fair and (ii) to prepare a report that will be made available to the shareholders of Erytech and Pherecydes (the “Merger Auditor”).
The reports of the Merger Auditor dated May 15, 2023 are attached as ANNEX 2 of the Exemption Document, and are made available to the shareholders of Erytech and Pherecydes.
The reports of the Merger Auditor have also been filed with the registry of the Commercial Courts of Lyon and Nantes in accordance with the regulations in force.
| 1.4 | INFORMATION SOURCED BY A THIRD PARTY |
The Exemption Document contains information relating to the companies’ activities and the markets in which they operate. This information is derived from studies carried out either by internal or external sources (e.g. industry publications, specialized studies, information published by market research companies, analysts’ reports). Other information contained in the Exemption Document is publicly available information. The companies believe that this information provides an accurate picture of their reference markets and their competitive position in these markets.
To the best of the companies’ knowledge, such information has been accurately reproduced and no facts have been omitted that would make it inaccurate or misleading. However, this information has not been verified by an independent expert and the companies cannot guarantee that a third party using different methods to collect, analyze or calculate market data would obtain the same results.
| 2. | INFORMATION ON THE ISSUER AND ON THE ABSORBED COMPANY |
| 2.1 | INFORMATION ON ERYTECH, THE ABSORBING COMPANY |
| 2.1.1 | General information |
| 2.1.1.1 | Commercial name, registered office, date of incorporation, law, legal form and legal entity identifier |
ERYTECH Pharma is a public limited liability company incorporated under the laws of France on October 26, 2004, having its registered office at 60, avenue Rockefeller, 69008 Lyon and registered with the Lyon Trade and Companies Register under number 479 560 013.
Legal Entity Identifier (LEI): 969500U8ZZCODU8A9374
Website: http://www.erytech.com/ - The contents of this website are not part of the Exemption Document, unless expressly incorporated by reference.
Phone number: + 33 4 78 74 44 38
| 2.1.1.2 | Statutory auditors |
Statutory Auditors
8
KPMG S.A., public limited liability, Nanterre Trade and Companies Register 775 726 417, 2 Avenue Gambetta Tour Eqho, Paris la Défense 92066 Nanterre Cedex.
Date of first appointment: June 24, 2016.
Expiry date of the mandate: General Meeting of shareholders approving the financial statements of the financial year ending December 31, 2027.
KPMG Audit Rhône Alpes Auvergne has been the statutory auditor since June 11, 2010 and until its replacement by KPMG S.A. on June 24, 2016 at the end of its mandate.
RSM Paris S.A.S., a simplified joint stock company, Paris Trade and Companies Register 792 111 783, 26, rue Cambacérès, 75008 Paris.
Date of first appointment: June 21, 2019.
Expiry date of the mandate: General Meeting of shareholders approving the financial statements of the financial year ending December 31, 2025.
RSM Rhône Alpes has been the statutory auditor since June 17, 2014 and until its replacement by RSM Paris on June 21, 2019.
| 2.1.2 | Business overview |
| 2.1.2.1 | Principal activities |
Erytech is a clinical-stage biotechnology company, founded in 2004, which develops innovative therapies, resulting from the internal conduct of research and development programs, to cure patients suffering from diseases in therapeutic areas whose needs are not currently met and which are based in particular on red blood cells.
The principal activities of Erytech are presented in Sections 1.1 “Présentation Générale”, 1.2 “Stratégie du Groupe”, 1.3 “Plateformes technologiques”, 1.4 “Tableau des produits en développement”, 1.5 “Eryaspase, une approche unique du traitement en oncologie” and 1.6 “Autres programmes thérapeutiques potentiels de la société” of the Erytech 2022 Universal Registration Document.
9
The table below set forth Erytech’s products candidates pipeline that will evolve following the final completion of the Merger:
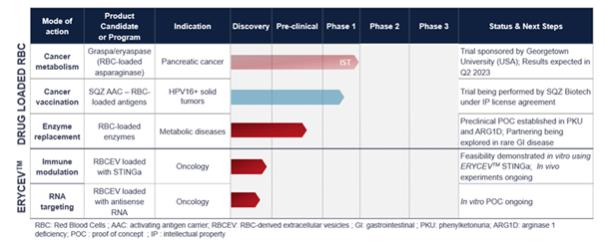
| 2.1.2.2 | Important events concerning the activities |
Any significant changes affecting Erytech’s operations and principal activities that have occurred since December 31, 2022 are described in Section 1.1 “Présentation Générale” of the Erytech 2022 Universal Registration Document.
| 2.1.2.3 | Principal markets |
Erytech’s principal markets are described in Section 1.8 “Commercialisation” of the Erytech 2022 Universal Registration Document.
| 2.1.3 | Investments |
None.
| 2.1.4 | Corporate Governance |
| 2.1.4.1 | Administrative and management bodies |
The administrative and management bodies of Erytech are described in Section 3.1.1.2 “Organes d’administration et de direction” of the Erytech 2022 Universal Registration Document.
10
As of the date of the Exemption Document, the composition of the board of directors of Erytech is as follows:
| Name, first name, |
1st nomination |
Term of mandate |
Independant |
Audit |
Clinical |
Compensation |
Experience of | |||||||
| Jean-Paul Kress French Chairman of the board of directors 57 y.o 50 Gray Street Boston
|
General Meeting of June 21, 2019 and Board of directors of June 21, 2019 | Ordinary General Meeting to be held in 2025 to approve the financial statements for the year ending December 31, 2024.
|
Yes | NA | NA | Member | The experience of the following directors is presented in Section 3.1.1.2.3 of the Erytech 2022 Universal Registration Document: Jean-Paul Kress; Gil Beyen; Sven Andréasson; Philippe Archinard; Martine Ortin George; Hilde Windels BV, represented by Hilde Windels. | |||||||
| Gil Beyen Belgian Director and Chief Executive Officer 61 y.o 96 South ST #4, Boston, MA 02111 (United-States)
|
General Meeting of April 2, 2013 (having been Chairman of the Supervisory Board since 2012) |
Ordinary General Meeting to be held in 2025 to approve the financial statements for the year ending December 31, 2024.
|
No | NA | NA | NA | ||||||||
| Sven Andréasson Swedish Director 70 y.o 3528 Reservoir Road NW, Washington D.C 20007 (United-States) |
Cooptation at the meeting of the Board of directors of January 4, 2022 (Chairman of the Supervisory Board from 2009 to 2011, Vice-Chairman of the Supervisory Board since 2011)
|
Ordinary General Meeting to be held in 2025 to approve the financial statements for the year ending December 31, 2024. |
Yes | Member | NA | Member | ||||||||
| Philippe Archinard French Director 63 y.o 47 rue Professeur |
General Meeting of April 2, 2013 (Member of the Supervisory Board since 2005) |
Ordinary General Meeting to be held in 2025 to approve the financial statements for the year ending December 31, 2024.
|
Yes | Member | Member | Member and Chairman | ||||||||
| Martine Ortin George French Director 74 y.o 24 Albert way Princeton, NJ 08540 (United-States)
|
General Meeting of June 17, 2014 | General Meeting of Shareholders to be held in 2023 to approve the financial statements for the year ending December 31, 2022.
|
Yes | NA | Member and Chairman | NA | ||||||||
| Hilde Windels BV represented by Hilde Windels Belgian Director 57 y.o Kasteellaan 89 9000 Gent (Belgium)
|
General Meeting of June 27, 2017 | General Meeting of Shareholders to be held in 2023 to approve the financial statements for the year ending December 31, 2022. | Yes | Member and Chairman | NA | NA | ||||||||
| Didier Hoch French Director 66 y.o 1508 route de |
Cooptation at the meeting of the Board of directors of May 15, 2023 ratified by the General Meeting of June 23, 2023 | The experience of the following directors is presented in Chapter 2 of the report on corporate |
11
| Name, first name, |
1st nomination |
Term of mandate |
Independant |
Audit |
Clinical |
Compensation |
Experience of | |||||||
| GO Capital, SAS represented by Mrs. Leila Nicolas French Director 1 rue Louis Braille Hall-a-Cap Courrouze 35136 St Jacques de la Lande |
Cooptation at the meeting of the Board of directors of May 15, 2023 ratified by the General Meeting of June 23, 2023 | governance, included in the Pherecydes 2022 Annual Financial Report: Didhier Hoch, Go Capital, represented by Leïla Nicolas |
| 2.1.4.2 | Identity of major shareholders |
The identity of the major shareholders of Erytech is presented in Section 4.1 “Répartition du capital et des droits de vote” of the Erytech 2022 Universal Registration Document.
Since the publication of the Erytech 2022 Universal Registration Document, the Company has received the following threshold crossing declaration: “On April 14, 2023, Akkadian Partners (18 rue Robert Stümper, L-2557 Luxembourg), acting on behalf of the fund Akkadian Partners Fund, of which it ensures the management, declared that on April 13, 2023, it had exceeded the threshold of 5% of the Company’s share capital and that it held, on behalf of the said fund, 1,570,000 shares in the Company representing the same number of voting rights, i.e. 5.06% of the share capital and 4.83% of the voting rights.”
12
As of the date of the Exemption Document, the distribution of the share capital is as follows:
| May 15, 2023 | ||||||||||||||||
| SHARES | % of capital | Total voting rights | Voting rights percentage |
|||||||||||||
| MANAGEMENT & EMPLOYEES |
25830 | 0,08 | % | 37806 | 0,11 | % | ||||||||||
| Gil BEYEN |
4 840 | 0,01 | % | 7 308 | 0,02 | % | ||||||||||
| Jérôme BAILLY |
3 798 | 0,01 | % | 5 619 | 0,02 | % | ||||||||||
| Eric SOYER |
6 264 | 0,02 | % | 8 574 | 0,02 | % | ||||||||||
| Anne-Cecile FUMEY |
864 | 0,00 | % | 1 333 | 0,00 | % | ||||||||||
| Karine CHARTON |
400 | 0,00 | % | 695 | 0,00 | % | ||||||||||
| Other employees |
9 664 | 0,03 | % | 14 277 | 0,04 | % | ||||||||||
| INVESTISSEURS FINANCIERS/PE FUNDS |
1449246 | 4,25 | % | 2898492 | 8,14 | % | ||||||||||
| AURIGA Partners |
1018212 | 2,98 | % | 2036424 | 5,72 | % | ||||||||||
| RECORDATI ORPHAN DRUGS |
431 034 | 1,26 | % | 862 068 | 2,42 | % | ||||||||||
| Directors |
10 303 | 0,03 | % | 20 606 | 0,06 | % | ||||||||||
| GALENOS |
1 | 0,00 | % | 2 | 0,00 | % | ||||||||||
| Philippe ARCHINARD |
10 300 | 0,03 | % | 20 600 | 0,06 | % | ||||||||||
| Hilde WINDELS |
1 | 0,00 | % | 2 | 0,00 | % | ||||||||||
| Martine GEORGE |
1 | 0,00 | % | 2 | 0,00 | % | ||||||||||
| Luc DOCHEZ |
0 | 0,00 | % | 0 | 0,00 | % | ||||||||||
| Melanie ROLLI |
0 | 0,00 | % | 0 | 0,00 | % | ||||||||||
| Other shareholders |
42 655 | 0,13 | % | 74 219 | 0,21 | % | ||||||||||
| Shareholders inferior or equal to 0,5% |
42 655 | 0,13 | % | 74219 | 0,21 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| AK1 total |
3101745 | 9,09 | % | 3101745 | 8,71 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pool Guy Rigaud |
500 535 | 1,47 | % | 500 535 | 1,41 | % | ||||||||||
| Auriga IV Bioseeds |
1 542 675 | 4,52 | % | 1 542 675 | 4,33 | % | ||||||||||
| FPCI Ouest Ventures III |
1058535 | 3,10 | % | 1058535 | 2,97 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| SUB-TOTAL NOMINATIF |
4629779 | 13,57 | % | 6132868 | 17,22 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Treasury Shares—ERYP |
2 500 | 0,01 | % | 0 | 0,00 | % | ||||||||||
| Unidentified (flottant) |
29390681 | 86,14 | % | 29390681 | 82,51 | % | ||||||||||
| BVF Partners L.P. |
97 338 | 0,29 | % | 97 338 | 0,27 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| SUB-TOTAL PORTEUR* |
29490519 | 86,43 | % | 29488019 | 82,78 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total net |
34120298 | 100,00 | % | 35620887 | 100,0 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | On the basis of threshold declarations |
Elaia Partners, Go Capital and the pool of shareholders of Pherecydes represented by Mr. Guy Rigaud have declared that they intend, together with AURIGA Partners, to act in concert with respect to Erytech. To the best of the Company’s knowledge, there are no other actions in concert nor shareholder agreement.
| 2.1.4.3 | Number of employees |
The number of employees of Erytech is set forth in Section 1.13.1.1 “Personnel” of the Erytech 2022 Universal Registration Document.
As of the date of the Exemption Document, Erytech has 38 employees.
13
| 2.1.5 | Financial information |
| 2.1.5.1 | Annual financial statements for the twelve months preceding the publication of the Exemption Document |
The financial statements of Erytech as at December 31, 2022 and the corresponding auditors’ report are presented in Sections 5.3.3 “Comptes sociaux établis (normes françaises) pour l’exercice clos le 31 décembre 2022” and 5.3.4 “Rapport des commissaires aux comptes sur les comptes sociaux établis pour l’exercice clos le 31 décembre 2022” of the Erytech 2022 Universal Registration Document.
The consolidated financial statements as at December 31, 2022 of the Erytech Group and the corresponding auditors’ report are set out in Sections 5.3.1 “Comptes consolidés établis en normes IFRS pour l’exercice clos le 31 décembre 2022” and 5.3.2 “Rapport des commissaires aux comptes sur les comptes consolidés établis en normes IFRS pour l’exercice clos le 31 décembre 2022” of the Erytech 2022 Universal Registration Document.
| 2.1.5.2 | Accounting standards |
The financial statements of Erytech presented in the Erytech 2022 Universal Registration Document are prepared in accordance with accounting standards applicable in France.
The consolidated financial statements of the Erytech Group presented in the Erytech 2022 Universal Registration Document are prepared in accordance with the IFRS accounting standards adopted by the European Union.
| 2.1.5.3 | Significant changes since the end of the last financial year |
Any significant changes having an impact on Erytech’s operations and principal activities that have occurred since December 31, 2022 are described:
| • | in Section 5.3.6 “Changement significatif de la situation financière ou commerciale” of the Erytech 2022 Universal Registration Document; |
| • | in Section 2.1.7 of the Exemption Document for the press releases published after the date of availability of the Erytech 2022 Universal Registration Document. |
| 2.1.5.4 | Management report |
The management report is incorporated into the Erytech 2022 Universal Registration Document as per the cross-reference table appearing on pages iv and v of the Erytech 2022 Universal Registration Document.
| 2.1.6 | Legal and arbitration proceedings |
To the best of Erytech’s knowledge, there are no administrative, legal or arbitration proceedings (including any pending or threatened proceedings) that are likely to have or have had, in the 12 months prior to the date of the Exemption Document, a material effect on the financial position or profitability of Erytech.
14
| 2.1.7 | Summary of information disclosed under Regulation (EU) N° 596/2014 of the European Parliament and of the Council |
Announcements relating to changes in Erytech’s strategy following the FDA’s feedbacks:
| • | Announcement of the sale of the cell therapy manufacturing facility in the United States to Catalent for a total consideration of 44.5 million dollars and update on the progress of clinical programs |
On April 25, 2022, Erytech announced the sale of Erytech’s state-of-the-art commercial-scale cell therapy manufacturing facility in Princeton, New Jersey, for a total consideration of $44.5 million. Catalent proposed to retain the Erytech’s staff at the site, representing approximately 40 people. Erytech announced that it will retain its manufacturing site in Lyon, and its expertise and capabilities in manufacturing process science to continue innovating in cell therapy manufacturing.
Erytech also provided an update on the progress of its clinical programs (including the BLA application in hypersensitive ALL, based on results of NOPHO-sponsored Phase 2 trial and rESPECT, the Phase 1 investigator-sponsored trial in first-line metastatic pancreatic cancer).
| • | Announcement of the end of Graspa® application for the treatment of patients with ALL hypersensitivity to pegylated asparaginase |
The Company received feedback from the FDA on its iPSP, submitted in July 2022. On August 24, 2022, Erytech announced its decision, following the feedback from the U.S. Food and Drug Administration’s (FDA), to no longer seek approval for Graspa® for the treatment of patients with ALL with hypersensitivity to pegylated asparaginase. Erytech announced that it has appointed a specialized advisor to evaluate strategic options to leverage its ERYCAPS® platform with complementary assets and/or a broader corporate transaction. Multiple options are under review, and the Company expects to give further updates on these strategic initiatives in the fourth quarter of this year.
| • | Announcement of the sale of the U.S. cell therapy manufacturing facility to Catalent and the discontinuation of operations in preparation for the submission of an application for approval of Graspa® for the treatment of patients with Acute Lymphoblastic Leukemia (ALL) |
On September 12, 2022, during its financial update for the first half of 2022, Erytech confirmed the sale of its U.S. cell therapy manufacturing facility to Catalent.
Erytech also confirmed that it has stopped activities to submit an application for approval of Graspa® for the treatment of patients with hypersensitive ALL, following recent feedback from the FDA.
Erytech further announced the suspension of enrollment in the TRYbeCA-2 trial, which evaluates eryaspase in combination with gemcitabine and carboplatin chemotherapy, compared to chemotherapy alone, in metastatic TNBC (first and second lines), following the disappointing results of eryaspase in the TRYbeCA-1 trial in second-line pancreatic cancer. The trial’s Steering Committee met in September 2022 to review the results of the 25 evaluable patients. No clinical benefit was demonstrated, which could be attributed to the immature closure of the trial and the small number of patients.
15
| • | Announcement of the halt of the lead program Graspa and deep restructuring implementation announced on the occasion of the publication of the third quarter of 2022 results |
On November 21, 2022, Erytech, on the occasion of the publication of its quarterly results, announced the halt of the program Graspa after FDA feedback on envisaged BLA submission in hypersensitive ALL and following inconclusive results from clinical studies, its intention to focus on its most promising preclinical programs. As a result, Erytech has announced that it has undertaken a restructuring of its staff with the implementation of a job preservation plan and has obtained the approval of the labor authorities in September 2022. Combined with the approximately 40 people who transferred to Catalent after the sale of the Company’s manufacturing facility in Princeton, the global team size will be less than 25% compared to the start of the year 2022. Erytech retains its R&D team and its expertise in key functional areas to keep the ability to restart a pipeline of partnered development programs and maintain a fully operational dual listed company.
Announcements relating to the Merger project:
| • | Announcement of the proposed combination between Erytech and Pherecydes intending to create a global leader in extended phage therapy |
On February 15, 2023, Erytech and Pherecydes announced the strategic combination of their two companies in order to build on complementary expertise and capabilities of both companies to accelerate development of extended phage therapies for antimicrobial resistance, in particular via the phase II PhagoDAIR study conducted by PHERECYDES, as well as other anti-infective fields and therapeutic areas with high unmet medical needs.
The transaction, supported by key shareholders of each of Pherecydes and Erytech will result in former Pherecydes shareholders holding approximately 49% of the combined entityand will extend the combined company cash runway into third quarter of 2024, with a consolidated cash position of approximately 41 million euros as of December 31, 2022, and would enable funding of existing and novel programs through multiple clinical milestones.
| • | Announcement of first quarter 2023 results and update on the merger project with Pherecydes |
On May 9, 2023, Erytech published its first quarter 2023 results. As of March 31, 2023, Erytech had cash and cash equivalents totaling 30.5 million euros (approximately 33.7 million dollars), compared to 38.8 million euros as of December 31, 2022.
In addition, Erytech indicated that on May 1, 2023, Akkadian Partners had informed the Board of directors that it intended to oppose the project of merging with Pherecydes and take de facto control of Erytech with a view to pursue alternative acquisition projects with Erytech’s cash. In that context, the Management and Board of Erytech have reviewed and assessed the ideas of acquisitions projects mentioned by Akkadian, with the assistance of external financial and legal advisers. After due consideration, Erytech determined that these ideas, were not in the best interest of Erytech and its stakeholders, and remote from Erytech’s strategy and identity, with significant uncertainty and risks associated with these projects. Erytech, while confirming its strategic decision to merge with Pherecydes, will oppose any financial predation project which would not be in the best interest of the company and its stakeholders.
16
| • | Update on the announced combination with Pherecydes |
On May 16, 2023, Ertytech announced that it have entered into the Merger Agreement with Pherecydes on May 15, 2023. In addition, Erytech announced that Erytech’s board of directors approved on May 15, 2023, the contribution by Elaia Partners, Go Capital and a pool of Pherecydes shareholders represented by Mr. Guy Rigaud, of 827,132 Pherecydes shares to Erytech in consideration of 3,101,745 newly issued Erytech shares. The exchange ratio for the Contribution is the same as for the Proposed Merger. Furthermore, Didier Hoch and Go Capital (represented by Mrs Leila Nicolas) have been appointed by the board of directors of Erytech by way of cooptation. Finally, Erytech announced that it intends to file a request with the President of the Commercial Court of Lyon soliciting the appointment of an ad hoc agent (mandataire ad hoc) who will represent absent Erytech shareholders to ensure that the required quorum is satisfied and the shareholders’ general meeting is allowed to validly resolve on all resolutions set forth on the agenda.
Announcements relating to the approval of the transfer of Erytech’s listing to the Nasdaq:
| • | Announcement of the receipt by Erytech of the Nasdaq Stock Market LLC’s notice |
On October 13, 2022, Erytech announced that it received a “Notification Letter” from the Nasdaq dated October 7, 2022, indicating that, based upon a closing bid price of less than $1.00 per share for the Company’s ADS for the prior 30 consecutive business day period, the Company no longer satisfies Nasdaq Listing Rule 5450(a)(1). Erytech benefited from the applicable grace period of 180 days to regain compliance, or until April 5, 2023. In the event the Company does not regain compliance within the 180-day grace period, and it meets all other listing standards and requirements, the Company may be eligible for an additional 180-day grace period. Erytech confirmed that it intended to regain compliance within the applicable compliance period and to evaluate its options to do so. During this time, the Company’s ADSs will continue to be listed and trade on The Nasdaq Global Select Market and the Company’s business and operations are not affected by the receipt of the Notification Letter.
| • | Announcement of the approval of the transfer of Erytech’s listing to the Nasdaq Capital Market |
On April 17, 2023, Erytech announced that it received approval from the Nasdaq Stock Market LLC on April 12, 2023 to transfer the listing of its American Depositary Shares from the Nasdaq Global Select Market to the Nasdaq Capital Market. The transfer became effective at the opening of business on April 14, 2023.
In connection with the transfer to the Nasdaq Capital Market, Nasdaq granted the Company an additional 180-day period (or until October 2, 2023) to regain compliance with the requirement set forth in Nasdaq Listing Rule 5450(a)(1) that the bid price of the Company’s ADS meet or exceed $1.00 per ADS for at least ten consecutive business days. If at any time during this additional time period the closing bid price of the Company’s security is at least $1 per share for a minimum of 10 consecutive business days, Nasdaq will provide written confirmation of compliance and this matter will be closed.
17
| 2.2 | INFORMATION ON PHERECYDES, THE ABSORBED COMPANY |
| 2.2.1 | General information |
| 2.2.1.1 | Commercial name, registered office, date of incorporation, law, legal form and legal entity identifier |
Pherecydes Pharma is a public limited liability company incorporated under the laws of France on December 12, 2006, having its registered office at 22, boulevard Benoni Goullin, 44200 Nantes, and registered with the Nantes Trade and Companies Register under number 493 252 266.
Legal Entity Identifier (LEI): 894500LYT3UUN58X3I68
Website: http://www.pherecydes-pharma.com/ - The contents of this website are not part of the Exemption Document, unless expressly incorporated by reference.
Phone number: +33 1 84 86 16 13
| 2.2.1.2 | Statutory auditors |
Statutory Auditor
PricewaterhouseCoopers Audit, a simplified joint stock company, Nanterre Trade and Companies Register 672 006 483, 63 Rue de Villiers, 92200 Neuilly-sur-Seine.
Date of first appointment: December 12, 2006.
Expiry date of the mandate: General Meeting of shareholders approving the financial statements of the financial year ending December 31, 2025.
Alternate statutory auditor :
Mr. Patrice Morot, 63 Rue de Villiers, 92200 Neuilly-sur-Seine.
Date of first appointment: May 28, 2021.
Expiry date of the mandate : General Meeting of shareholders approving the financial statements of the financial year ending December 31, 2025.
| 2.2.2 | Business overview |
| 2.2.2.1 | Principal activities |
Pherecydes is a clinical-stage biotechnology company, created in 2006, which develops innovative treatments, resulting from the internal conduct of research and development programs, to cure patients suffering from diseases in therapeutic areas whose needs are not currently met and which are based in particular on bacteriophages.
18
The principal activities of Pherecydes are presented in Chapter 1 “Activité de la société et évolution des affaires au cours de l’exercice clos le 31 décembre 2022” of the Pherecydes 2022 Annual Financial Report, in particular in Sections 1.1 “Présentation Générale de l’activité de la Société”, 1.2 “Situation de l’activité et analyse de l’évolution des affaires au cours de l’exercice 2022” and 1.4 “Activité en matière de R&D”.
The table set forth below presents Pherecydes’ product candidate pipeline, which will evolve following the completion of the Merger:

| 2.2.2.2 | Important events concerning the activities |
Any significant changes having an impact on Pherecydes’ operations and principal activities that have occurred since December 31, 2022 are described:
| • | in Section 1.7 “Evènements importants survenus entre la date de clôture et la date d’établissement du rapport” of the Pherecydes 2022 Annual Financial Report; |
| • | in Section 2.2.7 of the Exemption Document for the press releases published after the date of availability of the Pherecydes 2022 Annual Financial Report. |
| 2.2.2.3 | Principal markets |
Pherecydes focuses its activity on the development of treatments against three specific bacterial species identified by the WHO as the bacteria for which new treatments are most urgently needed and which together account for more than two thirds of the incidence of antibiotic-resistant infections, namely
| • | Staphylococcus aureus (S. aureus); |
| • | Pseudomonas aeruginosa (P. aeruginosa); and |
| • | Escherichia coli (E. coli). |
19

According to the U.S. Centers for Disease Control and Prevention (CDC), the three bacterial infections targeted by Pherecydes alone accounted for 3.7 billion dollars in healthcare costs in 20171.
In its report on 2020, the ECDC estimates that the health cost is about 1.8 billion euros. According to this report, the most common bacterium in Europe is E. coli (44.2%), followed by S. aureus (20.6%). P. aeruginosa comes in 5th position with a rate of 5.6%. Applying the above percentages, the health cost in Europe would therefore be 1.3 billion euros for S. aureus, E. coli and P. aeruginosa alone, the bacteria targeted by Pherecydes.
The first bacterium against which Pherecydes develops treatments is the S. aureus bacterium. This bacterium is resistant to methicillin and is recognized as one of the most dangerous pathogens, classified as a serious threat by the CDC and priority 2 out of 3 “high” by the WHO. It is a common bacterium that spreads in healthcare facilities and the general population.
Pherecydes has selected two active phages to treat infections caused by this bacterium in three different clinical indications, namely osteoarticular prosthesis infections (OAI), diabetic foot ulcers (DFU) and infective endocarditis (IE, heart valve infections).
The second bacterium against which Pherecydes is developing treatments is P. aeruginosa, recognized as one of the most dangerous pathogens, classified as a serious threat by the CDC and priority 1 of 3 “critical” by the WHO. P. aeruginosa causes many types of healthcare-associated respiratory infections (nosocomial diseases), including pneumonia, bloodstream infections, and surgical site infections.
| 1 | https://www.marketresearchfuture.com/reports/hospital-acquired-infections-market-2576 |
20
Pherecydes has selected four phages active on the P. aeruginosa bacterium with a focus on a pulmonary indication: ventilator-associated pneumonia (VAP), and potentially mucovisidosis (CF, Cystic Fibrosis).
Finally, the third bacterium against which Pherecydes is developing treatments is E. coli, also recognized as one of the most dangerous pathogens, also classified as a serious threat by the CDC and priority 1 out of 3 “critical” by the WHO. E. coli is a digestive tract bacterium of the Enterobacteriaceae family, frequently responsible for infections in human health (UTIs, especially complex urinary tract infections) and in animal health. This bacterium is easily transmitted when hygiene measures are insufficiently respected.
Pherecydes has selected four phages active on E. coli bacteria targeting complicated urinary tract infections (CTI).
The impact of these different clinical positions in Europe (5) and the United States is described thereafter.

| 2.2.3 | Investments |
Pherecydes has not made any material investments since December 31, 2022 that are in progress and/or for which firm commitments have been made.
21
| 2.2.4 | Corporate governance |
| 2.2.4.1 | Administrative and management bodies |
The administrative and management bodies of Pherecydes are described in chapter 1 of the Pherecydes 2022 Corporate Governance Report:
| Name |
Mandate | Age | Independent member (1) |
Member of the Audit Committee |
Member of the Compensation Committee |
Date of entry on the Board of directors |
Expiry date of the current mandate |
|||||||||||||||||||
| Didier Hoch |
Chairman of the Board of directors |
66 | 2022 | 2026 | ||||||||||||||||||||||
| Maryvonne Hiance |
Director | 74 | X | 2022 | 2026 | |||||||||||||||||||||
| Go Capital – represented by Leila Nicolas |
Director | 42 | X | 2022 | 2026 | |||||||||||||||||||||
| Elaia Partners reprensented by Franck Lescure, Director |
Director | 54 | 2022 | 2026 | ||||||||||||||||||||||
| Guy Rigaud |
Director | 75 | X | 2022 | 2026 | |||||||||||||||||||||
| Robert Sebbag |
Director | 72 | X | 2022 | 2026 | |||||||||||||||||||||
| Eric Leire |
Director | 65 | X | X | 2022 | 2026 | ||||||||||||||||||||
| (1) | With regard to recommendation no. 9 of the Middlenext corporate governance code for small and mid-sized companies of September 2021. |
The composition of the administrative and management bodies of Pherecydes have not changed since December 31, 2022.
| 2.2.4.2 | Identity of major shareholders |
The identity of the major shareholders of Pherecydes is presented in Section 6.1 of the Pherecydes 2022 Annual Financial Report.
| Shareholder |
Number of shares |
% of share capital |
Number of voting rights |
% voting rights | ||||||||||||
| Member of the board of directors and of the management* |
4 483 | 0.06 | % | 4 483 | 0.06 | % | ||||||||||
| ACE |
1 384 564 | 17.44 | % | 1 384 564 | 17.44 | % | ||||||||||
| Omnes Capital |
242 598 | 3.06 | % | 242 598 | 3.06 | % | ||||||||||
| Participations Besançon |
243 819 | 3.07 | % | 243 819 | 3.07 | % | ||||||||||
| Treasury shares |
25 142 | 0.32 | % | 25 142 | 0.32 | % | ||||||||||
| Auriga IV Bioseeds |
1 436 977 | 18.10 | % | 1 436 977 | 18.10 | % | ||||||||||
| Ouest Venture III |
986 009 | 12.42 | % | 986 009 | 12.42 | % | ||||||||||
| Pool GR |
466 369 | 5.87 | % | 466 369 | 5.87 | % | ||||||||||
| Erytech |
827 132 | 10.42 | % | 827 132 | 10.42 | % | ||||||||||
| Floating capital |
2 322 086 | 29.25 | % | 2 322 086 | 29.25 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
7 939 179 | 100.00 | % | 7 939 179 | 100.00 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | Given the termination of the functions of Mr. Guy-Charles Fanneau de La Horie, previously Chairman of the Board of Directors, on May 23, 2022, and of Mr. Philippe Rousseau, previously a member of the Board of directors, on March 31, 2022, the shares of the Company that they hold as of December 31, 2022 are not included in this number of shares. This number corresponds to the shares held personally by Mr. Guy Rigaud, not included in the “Pool GR” line below, and by Mr. Didier Hoch, Chairman of the Board of Directors. The shares owned by Elaia Capital and Go Capital are shown in the corresponding lines of the table. |
22
Pherecydes holds 25,142 of its own shares as of the date hereof, i.e. 0.32% of its share capital, under a liquidity contract entrusted to Portzamparc. This contract has been temporarily suspended at the end of the trading session on May 5, 2023.
In addition, the shareholders of the Guy Rigaud Pool entered into a voting agreement on December 22, 2017. This voting agreement entails concerted action.
| 2.2.4.3 | Number of employees |
Pherecydes currently employs 29 people (full-time employees).
| 2.2.5 | Financial information |
| 2.2.5.1 | Annual and half-yearly financial statements for the twelve months preceding the publication of the Exemption Document |
The financial statements of Pherecydes as at December 31, 2022 (pages 67 et seq.) and the corresponding auditor’s report (pages 91 et seq.) are presented in the appendix to the Pherecydes 2022 Annual Financial Report.
| 2.2.5.2 | Accounting standards |
The financial statements of Pherecydes presented in the Pherecydes 2022 Annual Financial Report have been prepared in accordance with accounting standards applicable in France.
| 2.2.5.3 | Significant changes since the end of the last financial year |
Any significant changes in the financial position of Pherecydes since December 31, 2022 are described:
| • | in Section 1.7 “Evènements importants survenus entre la date de clôture et la date d’établissement du rapport” and in Section 3.2 of the notes to the financial statements for the year ended December 31, 2022 included in the Pherecydes 2022 Annual Financial Report; |
| • | in Section 2.2.7 of the Exemption Document for the press releases published after the date of availability of the Pherecydes 2022 Annual Financial Report. |
| 2.2.5.4 | Management report |
The management report is incorporated into the Pherecydes 2022 Annual Financial Report in the first part of the document (pages 3 et seq.).
23
| 2.2.6 | Legal and arbitration proceedings |
To the best of Pherecydes’ knowledge, there are no administrative, legal or arbitration proceedings (including any pending or threatened proceedings), that are likely to have or have had, in the 12 months prior to the date of the Exemption Document, a material effect on the financial position or profitability of Pherecydes.
| 2.2.7 | Summary of information disclosed under Regulation (EU) N° 596/2014 of the European Parliament and of the Council |
Elements related to the activity
| • | Announcement of the first international approval of a compassionate treatment with its phages—The Swedish Medical Products Agency (SMPA) has given its approval to treat a compassionate case of osteoarticular infection on prosthesis joint with anti-S. aureus phages |
On April 28, 2022, Pherecydes announced that it had received the first international approval of a compassionate treatment with its phages, in Sweden. The Swedish Medical Products Agency (SMPA) has given its approval to treat a case of osteoarticular infection of a prosthetic joint with Pherecydes Pharma’s anti-S. aureus phages.
| • | Announcement of the obtention of the Compassionate Access Authorization (AAC) Early Access Program approval from the ANSM (Agence Nationale de Sécurité du Médicament et des produits de santé) for its anti-Staphylococcus aureus phages |
On May 30, 2022, Pherecydes announced that it had been granted Compassionate Access Authorization (AAC) Early Access Program from the ANSM (Agence Nationale de Sécurité du Médicament et des produits de santé, the French National Agency for the Safety of Medicines and health products) for its anti-Staphylococcus aureus (S. aureus) phages.
The AAC system allows certain categories of sick patients in France with no therapeutic solutions to benefit from drugs yet to be granted marketing approval. The AAC scheme allows Pherecydes Pharma to make its anti-S. aureus phages available to larger populations and thus generate the first revenue in the Company’s history.
| • | Announcement of the enrollment of the first patient in the phase II study PhagoDAIR for the treatment of osteoarticular infections caused by Staphylococcus aureus |
On June 15, 2022, Pherecydes announced the enrollment of the first patient in the phase II study PhagoDAIR.
PhagoDAIR is the world’s first phage therapy study conducted in osteoarticular infections on prostheses caused by Staphylococcus aureus (S. aureus). Its protocol has been approved by the French National Agency for the Safety of Medicines and health products (ANSM) in December 2021 and by the French Committee for the Protection of Individuals (CPP) in February 2022.
24
| • | Announcement of positive preclinical results of inhaled phage therapy presented to the Reanimation 2022 conference |
On June 27, 2022, Pherecydes announced that the results of a preclinical study undertaken with its phages have been presented at the Reanimation 2022 conference organized by the SRLF (Société de Réanimation de Langue Française, the French intensive care society) and held in Paris from June 22 to 24, 2022.
The study was carried out within the framework of the Pneumophage project, associating the UMR1100 and the Diffusion Technique Française company, aimed at demonstrating the effectiveness of inhaled phage therapy in treating ventilator-associated infections.
The results obtained also demonstrate the feasibility of delivering large quantities of active phages by nebulization during mechanical ventilation and the rapid control of the infection in situ in a respiratory model close to humans.
| • | Announcement of a first registration of the phagogram as an in vitro diagnostic test in accordance with EC Directives |
On September 12, 2022, Pherecydes Pharma announced the registration of its phagogram as an in vitro diagnostic test (“Phagogram 1.5”) in accordance with Directive 98/79/EC.
The phagogram is an in vitro diagnostic test to verify the sensitivity of patients’ bacterial strains to Pherecydes phages.
| • | Announcement of the creation of an international Medical Advisory Board |
On September 15, 2022, Pherecydes announced the setting up of a Medical Advisory Board comprising prominent international scientific and clinical experts in infectious diseases. This Board will support Pherecydes consolidate its clinical development strategy in phage therapy.
| • | Announcement of positive recommendation of the DSMB for the continuation of its phase II clinical study PhagoDAIR in osteoarticular infections caused by Staphylococcus aureus |
On November 29, 2022, Pherecydes announced that it had received a unanimous recommendation from the Data Safety Monitoring Board (DSMB) to continue without modification the phase II clinical study PhagoDAIR in osteoarticular infections of prostheses caused by Staphylococcus aureus (S. aureus).
The DSMB is an independent expert committee responsible for the open-label review of the PhagoDAIR study safety data and will meet twice a year during the study. Following its first meeting, the committee recommended that the study continue without modification.
| • | Announcement of the presentation of Phagogram 1.5 at the ECCMID congress |
On April 15, 2023, Pherecydes presented part of its analytical performance plan adapted to its Phagogram 1.5 in vitro diagnostic test to verify the sensibility of patients’ bacterial strains to Pherecydes phages, at the 33rd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) in Copenhagen (Denmark).
25
Changes in governance
| • | The general assembly of Pherecydes approves the evolution of its governance towards a company with a Board of Directors—Appointment of Didier Hoch as Chairman and CEO and Thibaut du Fayet as Deputy CEO |
On May 23, 2022, Pherecydes announced that the Combined General Meeting held on May 19, 2022 approved the change of the company’s management and administration by adopting a Board of Directors and a General Management.
The current members of the Supervisory Board were appointed as the first directors of Pherecydes. Mr. Didier Hoch was appointed Chairman and CEO and Mr. Thibaut du Fayet was appointed Deputy CEO.
| • | Separation of the functions of Chief Executive Officer and Chairman of the Board of Directors |
On December 15, 2022, following the Combined General Meeting of Pherecydes shareholders, the Board of Directors adopted the following decisions: the functions of Chief Executive Officer and Chairman of the Board of Directors were separated, Mr. Thibaut du Fayet, previously Deputy Chief Operating Officer, was appointed Chief Executive Officer and Mr. Didier Hoch, previously Chairman and Chief Executive Officer, was appointed Chairman of the Board of Directors.
Significant financial transactions and announcement of the Merger
| • | Announcement of a capital increase of approximately 3 million euros to pursue its clinical development program in phagotherapy |
On September 22, 2022, Pherecydes announced the success of its capital increase, launched on September 21, 2022, for a total amount of 3.1 million euros, of which 2.6 million euros from institutional investors and 0.5 million euros from individuals.
| • | Announcement of the proposed combination between Erytech and Pherecydes to create global leader in extended phage therapies |
On February 15, 2023, Erytech and Pherecydes announced the combination of their two companies (please refer to Section 2.1.7 above for more details on the communication made).
| • | Announcement of the completion of a 1.5 million euros capital increase reserved to its historical shareholders |
On February 17, 2023, Pherecydes announced the completion of the capital increase for a total amount of 1.5 million euros, fully subscribed by Auriga IV Bioseeds, Ouest Ventures III2 and the pool of shareholders represented by Mr. Guy Rigaud, historical shareholders of Pherecydes.
| 2 | See section 3.2.1.6 of this Exemption Document for a description of the relationship between Auriga IV Bioseeds, Ouest Ventures III and Erytech. |
26
The funds raised through this capital increase will provide Pherecydes Pharma with sufficient resources to finance its cash requirements until the completion of the merger, expected no later than June 30, 2023.
| • | Announcement of 2022 annual financial results |
On March 30, 2023, Pherecydes presented its 2022 annual financial results. As of December 31, 2022, Pherecydes had cash and cash equivalents of 1 million euros (approximately 1.1 million dollars), compared to 5.4 million euros as of December 31, 2021. In addition to this cash, the capital increase completed on February 17, 2023 for a total amount of 1.5 million euros, is part of the merger project of Pherecydes Pharma into Erytech Pharma announced on February 15, 2023. The current cash position allows Pherecydes Pharma to cover its cash needs until the end of the first half of 2023, when the merger with Erytech is expected.
| • | Pherecydes strengthens its corporate links with Erytech to prepare the strategic combination |
On May 16, 2023, Pherecydes announced the entering into the Merger Agreement with Erytech on May 15, 2023. In addition, Pherecydes announced that the contribution by Elaia Partners, Go Capital and a pool of Pherecydes shareholders represented by Mr. Guy Rigaud, of 827,132 Pherecydes shares to Erytech in consideration of 3,101,745 newly issued Erytech shares has been approved by Erytech’s board of directors on May 15, 2023, which also approved the appointment of Mr. Didier Hoch and Ms. Leila Nicolas, representing Go Capital, as directors.
| 3. | DESCRIPTION OF THE MERGER |
| 3.1 | PURPOSE AND OBJECTIVES OF THE MERGER |
| 3.1.1 | Purpose of the Merger for the Absorbing Company and its shareholders |
The Merger is part of a strategic combination aimed at creating a global leader in phage therapy by capitalizing on the financial resources and teams of both the Absorbed Company and the Absorbing Company to both accelerate and expand Pherecydes’ existing phage therapy development programs, launch new phage candidates and potentially broaden the scope of application to new therapeutic modalities by leveraging both companies’ advanced technology platforms and expertise.
The Merger would close the strategic evaluation process announced by Erytech on several occasions since November 2021, and would represent the outcome of its effort to find strategic alternatives and a new direction for Erytech. Following the failure of its phase 3 trial in pancreatic cancer, Erytech has sought to leverage its corporate structure and capabilities by adding a clinical stage asset to its business in an area of significant unmet need. Antibiotic resistance is a major medical challenge worldwide and the phage therapy programs developed by Pherecydes represent a promising approach to targeting pathogenic bacteria such as S. aureus, E. coli and P. aeruginosa, which together are responsible for more than 800,000 resistant infections per year in the United States and Europe.
Erytech’s capabilities, expertise and assets, and in particular its late-stage clinical oncology experience, would complement and reinforce the efforts of Pherecydes’ teams to help create value. As part of the Merger, it is planned to relocate all teams to Erytech’s premises in Lyon, France, where they will benefit from a location within a major European center in the field of infectious diseases.
27
| 3.1.2 | Purpose of the Merger for the Absorbed Company and its shareholders |
Erytech and Pherecydes are expected to combine key expertise and capabilities to drive Pherecydes’ research and development programs, an experienced and highly complementary management team, and an international position that provides access to U.S. investors and stakeholders, particularly given Erytech’s listing on Nasdaq. These assets will allow to accelerate the clinical development plan of Pherecydes with international randomized controlled studies, aiming at establishing the clinical proof of concept of phagotherapy.
The shareholders of Pherecydes will receive, in the context of the Merger, Erytech shares, according to an exchange ratio of four (4) shares of Pherecydes for fifteen (15) shares of Erytech (determined as described in Section 3.5 of the Exemption Document). Upon completion of the Merger, the shareholders of Pherecydes will hold approximately 49% of the share capital and voting rights of Erytech3. Please refer to Section 5.5.2 of the Exemption Document for more details.
| 3.1.3 | Description of the anticipated benefits resulting from the Merger |
The Merger will allow to benefit from the complementarity between the resources offered by Erytech and the expertise of Pherecydes, with regard to the opportunities represented by the phage market.
Thanks to Erytech’s current cash position (38.8 million euros as of December 31, 2022), the financial visibility of the Absorbing Company, following the completion of the Merger, would extend to the 3rd quarter of 2024, with a consolidated unaudited cash position of approximately 41 million euros by December 31, 2022, and would fund multiple clinical milestones in its existing and future programs.
Other potential complementarities and synergies brought by Erytech are:
| • | A process and infrastructure at an advanced stage of development, and R&D and production capabilities potentially complementary to those of Pherecydes; |
| • | A presence and experience in the United States, notably through its dual listing on Nasdaq and Euronext Paris. |
Pherecydes would bring its ambitious clinical development plan in antibiotic resistance, including a phase II study and several phase I/II clinical studies, and other potential activities: development beyond antibiotic resistance (One Health, cosmetics) and phagogram (IVD diagnostic test).
| 3 | On the basis of the share capital of Pherecydes and Erytech diluted only with the respective instruments (mainly BSCPE and free shares) in the currency and taking into account the capital increase of Pherecydes completed on February 17, 2023 |
28
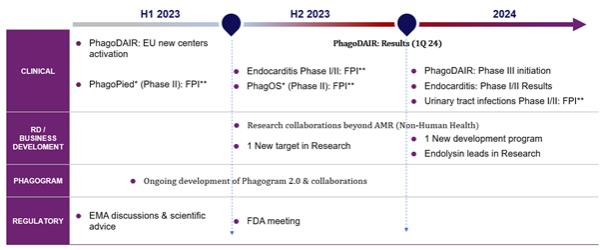
The goal of the new entity in 2023 and 2024 would be to focus on the following objectives:
| • | Expansion of the ongoing phase II PhagoDAIR study in patients suffering from Staphylococcus aureus (S. aureus) infections of knee or hip joints, by opening new clinical centers in Europe, with results expected in the first half of 2024. |
| • | Expansion of Pherecydes’ clinical portfolio in phage therapy with two additional phase II studies funded by the company, one in patients with S. aureus endocarditis, which is expected to start in mid-2023, and the second in patients with complex urinary tract infections due to Escherichia coli (E. coli), which is expected to start in the 1st quarter of 2024. |
| • | Development of a research and development strategy based on Erytech’s platforms and expertise, including drug delivery solutions using red blood cells (ERYCAPS) or red blood cell derived vesicles (ERYCEV), formulation expertise in oncology to support phage and endolysin-based therapeutic approaches in anti-infective areas such as antibiotic resistance and beyond, such as food, cosmetics and animal health, or the development of new carriers. |
| • | Extension of the Pherecydes portfolio to include two new phages complementary to the three already existing (S. aureus, P. aeruginosa, E. Coli), essential to develop a complete clinical portfolio of targets in the fight against resistant bacterial infections. |
| • | Capitalize on Erytech’s presence in the United States to facilitate access to North American investors and clinical and regulatory players for future clinical development. |
The pipeline of product candidates following the completion of the Merger is presented below:
| 3.2 | CONDITIONS OF THE MERGER |
This Section includes the main elements required by Article 91 (2) of Directive (EU) 2017/1132 contained in the Merger Agreement, a copy of which is reproduced in full in ANNEX 1 of the Exemption Document.
29
| 3.2.1 | Legal aspects of the Merger |
| 3.2.1.1 | Legal framework of the Merger |
The Merger consists of a merger of Pherecydes into Erytech. The Merger Agreement is governed by French law and in particular by articles L. 236-1 et seq. of the French Commercial Code.
No special benefits are granted in accordance with the provisions of Article 91(2) of Directive (EU) 2017/1132.
| (a) | Date of the meeting of the governance bodies having approved the Merger operation |
The boards of directors of Erytech and Pherecydes have agreed on the terms of the Merger Agreement by decision dated May 5, 2023.
The Merger Agreement was signed on May 15, 2023.
| (b) | Closing date of the accounts used for the determination of the exchange ratio of the Merger |
The terms and conditions of the Merger are established on the basis of:
| • | with respect to the Absorbing Company: the annual accounts for the financial year ending on December 31, 2022, as approved by the Board of directors of the Absorbing Company on March 22, 2023 and certified by the statutory auditors on March 28, 2023, as set forth in Annex 6(a) to the Merger Agreement; and |
| • | with respect to the Absorbed Company: the annual accounts for the financial year ending on December 31, 2022, as approved by the Board of directors of the Absorbed Company on March 31, 2023 and certified by the statutory auditors on April 26, 2023, as set forth in Annex 6(b) to the Merger Agreement. |
| 3.2.1.2 | Retroactive date and completion date of the Merger, conditions precedent to the effectiveness of the Merger, including any guarantee |
| (a) | Effective date of the Merger from an accounting and tax point of view |
From an accounting and tax perspective, the Merger will be effective retroactively as of January 1, 2023 (the “Effective Date”) in accordance with the provisions of Article L. 236-4 of the French Commercial Code, so that all active and passive transactions carried out by the Absorbed Company from the Effective Date until the Completion Date (as defined below) will be deemed to have been carried out for the benefit of, or at the expense of, the Absorbing Company.
30
Thus:
| • | the assets and liabilities of the Absorbed Company will be transferred to the Absorbing Company in the state in which they will be on the Completion Date, as a result of the universal transfer of assets and liabilities, which, by express consent, will mean that the Absorbing Company will take over all the corporate operations, without any reservation, carried out by the Absorbed Company from the Effective Date until the Completion Date; and |
| • | the Absorbing Company will become debtor of the creditors of the Absorbed Company in place of the latter, without such substitution entailing novation in their respect. |
As the Merger involves the transfer of the universality of the assets of the Absorbed Company to the Absorbing Company, the contributions and the liabilities encumbering these contributions will relate to the generality of the elements composing the assets of the Absorbed Company, even if not specifically designated or omitted in the nomenclature established on the basis of the annual accounts of the Absorbed Company. As a result, this nomenclature is merely declarative and not restrictive.
| (b) | Date of completion of the Merger |
The Merger and the resulting dissolution of the Absorbed Company will only be completed on the date of satisfaction of the last of the conditions precedent set forth below (the “Completion Date”).
| (c) | Conditions precedent |
The Merger is subject to the following conditions precedent:
| • | the delivery by the statutory auditor of the Merger of (i) a report on the value of the contributions and (ii) a report on the terms and conditions of the Merger confirming the fairness of the exchange ratio retained; |
| • | the approval by the extraordinary shareholders’ meeting of (i) the Absorbed Company of the Merger, and of the resulting dissolution of the Absorbed Company, and (ii) the Absorbing Company, of the Merger, and of the corresponding capital increase of the Absorbing Company in consideration of the Merger; and |
| • | the approval by the Extraordinary General Meeting of the shareholders of the Absorbing Company of the resolutions relating to the appointment of the directors appointed by Pherecydes and the amendment of the articles of association of Erytech relating to the removal of the casting vote of the chairman of the Board of directors. |
In any event, it is specified that if the conditions precedent are not fulfilled before July 31, 2023 at midnight at the latest, the Merger Agreement will be automatically terminated, without any indemnity on either side, unless both parties waive the Merger Agreement.
31
| 3.2.1.3 | Main amendments to the articles of association of the Absorbing Company |
No modification of the articles of association is planned other than those resulting from the adaptation of the bylaws of the Absorbing Company as a consequence of the Merger, namely:
| • | the modification of the corporate name of the Absorbing Company provided for in article 2 of the articles of association of the Absorbing Company; |
| • | the addition of a paragraph summarizing the capital increase resulting from the Merger to article 6 of the articles of association of the Absorbing Company; |
| • | the update of the new amount of the share capital following the Merger as provided for in article 7 of the article of association of the Absorbing Company; |
| • | the deletion of the age limit for the duties of censor provided for in article 18 of the articles of association of the Absorbing Company; and |
| • | the deletion of the stipulation stating that in the event of a tie vote within the board of directors, the Chairman of the meeting shall have the casting vote, as provided for in article 19 of the articles of association of the Absorbing Company. |
3.2.1.4 Opinion of the social and economic committee of the Absorbing Company
The social and economic committee of Erytech has been informed about the Merger and has issued a favorable advisory opinion on the Merger on March 20, 2023.
| 3.2.1.5 | Tax regime of the Merger |
From a tax and accounting perspective, the Merger will take effect retroactively as of the Effective Date, i.e. on January 1, 2023.
From a corporate income tax perspective, the Merger is placed under the preferential regime of article 210 A of the French General Tax Code (Code general des impôts). To this end, Erytech has made, in the Merger Agreement, all the commitments provided for in the said article.
With regards to registration duties, in accordance with articles 635, 1-5° and 816 of the French General Tax Code, the Merger will be registered free of charge within a period of one month following the Completion Date. If applicable, the transfer of any title deed of real estate will however be subject, upon its registration, to a real estate security contribution at the rate of 0.1% on the market value of such real estate, in accordance with articles 879 and 881 K of the same code.
| 3.2.1.6 | Pre-existing links between the companies involved in the Merger |
| (a) | Capital links |
| • | Ownership of the Absorbed Company by the Absorbing Company |
On May 5, 2023, Elaia Partners (acting on behalf of Auriga IV Bioseeds), Go Capital (acting on behalf of Ouest Ventures III) and certain shareholders of the Absorbed Company led by Mr. Guy Rigaud, shareholders representing together approximately 46.814% of the share capital and voting rights of the Absorbed Company, together with the Absorbing Company, entered into a contribution in kind agreement pursuant to which they agreed to contribute, prior to the Completion Date, 827,132 ordinary shares of the Absorbed Company (the “Contributions in Kind”).
| 4 | Prior to the completion of the Contributions in Kind. |
32
These Contributions in Kind are made in accordance with the support commitments entered into in the context of the signature of the Memorandum of Understanding, pursuant to which these shareholders of the Absorbed Company have undertaken to contribute these shares before the Completion Date (as defined below in Section 3.2.1.2) in exchange for newly issued Erytech shares, at the same exchange ratio as the Merger.
A share capital increase of the Absorbing Company by issuance of 3,101,745 new Erytech shares as consideration for the contribution of the 827,132 ordinary shares of the Absorbed Company referred to above has been decided by the meeting of the Board of directors of the Absorbing Company held in May 15, 2023 in accordance with the delegation of powers granted by the general meeting of shareholders of the Absorbing Company held on June 24, 2022 (29th resolution)
Upon completion of the Contribution in Kind, Erytech holds 827,132 shares of Pherecydes, i.e. 10.42% of the share capital and voting rights of Pherecydes, and undertakes to maintain this shareholding unchanged until the Completion Date.
The report of the statutory auditors was filed with the clerk of the Commercial Court of Lyon in accordance with the regulations in force.
| • | Ownership of the Absorbing Company by the Absorbed Company |
The Absorbed Company does not hold any shares of the Absorbing Company to date.
| (b) | Other links |
| • | Common corporate officers |
At the close of the Board of directors’ meeting of May 15, 2023, the Board of Directors co-opted Mr. Didier Hoch and the company Go Capital, represented by Mrs. Leila Nicolas, as directors of the Absorbing Company.
| • | Guarantee |
As of the date of this Exemption Document, neither the Absorbing Company nor the Absorbed Company has guaranteed each other.
| • | Regulated agreements |
As of the date of this Exemption Document, no agreement has been entered into between the Absorbing Company and the Absorbed Company, except for the (i) Memorandum of Understanding and the (ii) Merger Agreement.
It should be noted that, at the date of its conclusion, the Contribution Agreement, did not constitute a regulated agreement within the meaning of article L. 225-38 of the French Commercial Code.
33
| 3.2.1.7 | Financial instruments giving access to the share capital of the Absorbed Company |
Pursuant to articles L. 225-197-1 and L. 228-98 to L. 228-106 of the French Commercial Code and article 6.3.2 of the regulations of the free share allocation plan adopted by the board of directors of the Absorbed Company on May 19, 2022, the Absorbing Company will automatically replace the Absorbed Company in its obligations towards the beneficiaries of free Pherecydes Shares and the beneficiaries of warrants for subscription to business creator shares (the “BSPCE”).
The rights of the beneficiaries will therefore be transferred to new shares of the Absorbing Company in application of the exchange ratio referred to in Section 3.2.3.2 below, according to the following formalities: the number of shares of the Absorbed Company to which each beneficiary would be entitled in the case of the same allocation plan will correspond to the number of shares of the Absorbing Company to which he would have been entitled under this plan multiplied by the exchange ratio applicable to the shareholders referred to in Section 3.2.3.2 below, the number thus obtained being rounded down to the nearest whole number. It is specified that the beneficiaries of BSPCE and free shares will be notified of the above conditions by the Absorbed Company, before the planned Completion Date.
The other provisions provided for by the regulations and plans for the allocation of free shares and BSPCE, and in particular the provisions relating to the vesting and retention periods, for their remaining duration on the Completion Date, remain applicable to the rights and allocations and to the new shares of the Absorbing Company received in exchange by the beneficiaries.
The extraordinary general meeting of the Absorbing Company called to rule on the Merger will take note of the obligations of the Absorbing Company resulting from this assumption of the commitments of the Absorbed Company and will waive the preferential subscription right to the ordinary shares that will be issued by the Absorbing Company as a result of the exercise of the BSPCE and the definitive allocation of the free shares.
| 3.2.2 | Merger control |
| 3.2.2.1 | Dates of the corporate bodies called to approve the Merger |
The approval of the Merger will be subject to the decision of the general meeting of the shareholders of Erytech and Pherecydes on June 23, 2023. Please refer to Section 4.3.2 “Resolutions, authorizations and approvals pursuant to which the securities will be created and/or issued” of this Exemption Document.
Auriga Partners (acting on behalf of Auriga Ventures III) and Recordati SpA, which together represent approximately 4.24 % of the share capital and 8.14 % of the voting rights of Erytech, have confirmed their support for the Merger and their commitment to vote in favor of the related resolutions.
Elaia Partners (acting on behalf of Auriga IV Bioseeds), Go Capital (acting on behalf of Ouest Ventures III) and certain shareholders of Pherecydes led by Mr. Guy Rigaud, representing together approximately 46.81% of the share capital and voting rights of the Absorbed Company, have contributed, on May 15, 2023, 827.132 Pherecydes shares to the Absorbing Company in consideration of newly issued 3,101,745 Erytech shares. These shareholders have committed to (i) in their capacity as shareholders of Erytech, vote in favor of the Merger project at the general meeting of shareholders of Erytech and (iii) in their capacity as shareholders of Pherecydes, vote in favor of the Merger project at the general meeting of shareholders of Pherecydes.
34
It is specified that, in accordance with Erytech’s press release dated May 16, 2023, that in view of the next general meeting of the shareholders of Erytech, Erytech intends to file a request with the President of the Commercial Court of Lyon to appoint an ad hoc agent (mandataire ad hoc).
The ad hoc agent will represent the shareholders absent at the General Meeting to ensure that the required quorum is satisfied and the shareholders’ general meeting is allowed to validly resolve on all resolutions set forth on the agenda.
In accordance with the terms of the order to be issued by the President of the Commercial Court of Lyon, the ad hoc agent will exercise the voting rights attached to the shares of the absent shareholders to the extent necessary to reach the required quorum, so that its vote will be neutral (proportion of 50% positive votes and 50% negative votes with respect to ordinary resolutions; proportion of 2/3 positive votes and 1/3 negative votes with respect to extraordinary resolutions). Consequently, the voting rights exercised by the ad hoc agent will have no impact on the outcome of the vote of the general meeting of shareholders of Erytech.
| 3.2.2.2 | Merger Auditor |
Ruling on the joint request of Erytech and Pherecydes, the President of the Commercial Court of Lyon, by order of February 28, 2023, has appointed the firm Finexsi as Merger Auditor, represented by Mr. Christophe Lambert.
Please refer to Section 1.3 “Expert’s Statement or Report” of the Exemption Document.
| 3.2.3 | Merger Consideration |
| 3.2.3.1 | Beneficiaries of Erytech actions |
The Erytech shares will be allocated to the Pherecydes shareholders as consideration for the Merger.
| 3.2.3.2 | Exchange ratio, amount of any cash payment and number of shares offered |
As consideration for the Merger, the Absorbing Company will issue 26,575,893 new shares based on the exchange ratio retained in the context of the Merger which is fifteen (15) shares of the Absorbing Company for four (4) shares of the Absorbed Company.
| 3.2.3.3 | Remuneration of contributions |
| (a) | Share capital increase |
The Absorbed Company holds 25,142 of its own shares as of the date hereof, i.e. 0.32% of its share capital.
Pursuant to the provisions of Article L. 236-3 II of the French Commercial Code, neither the exchange of the shares of the Absorbed Company that will be held by the Absorbing Company as a result of the Contributions in Kind (as defined in the Merger Agreement), nor the exchange of the treasury shares held by the Absorbed Company, which will be cancelled by operation of law on the Completion Date, will take place.
35
As consideration for the Merger, the Absorbing Company will proceed, on the Completion Date, pursuant to the exchange ratio, to an increase of its share capital by an amount of 2,657,589.30 euros, by the creation of 26,575,893 new shares having the same nominal value (i.e., 0.10 euro each) as the existing shares (the “New Shares”), which will be directly allocated to the shareholders of the Absorbed Company, other than the Absorbing Company, in accordance with the applicable exchange ratio.
It is specified that the final number of New Shares to be issued and, accordingly, the nominal amount of the resulting share capital increase will be adjusted by operation of law according to the exact number of Pherecydes shares to be remunerated pursuant to the Merger, in case, among other things, the amount of the New Shares to be issued and the nominal amount of the resulting share capital increase would be adjusted as a result of an inter-company transaction between the date of the Merger Agreement and the Completion Date.
No fractional shares will be issued. Each shareholder of the Absorbed Company will be credited with a number of ordinary shares of the Absorbing Company corresponding to the integer equal to or immediately lower than the product of the number of shares of the Absorbed Company that he/she will hold at the Completion Date by the exchange ratio provided in Article 14 of the Merger Agreement.
It is specified that the holders of shares of the Absorbed Company who do not own the necessary number of shares of the Absorbed Company to obtain a whole number of shares of the Absorbing Company will receive a cash payment for the fractional part. The amount of this cash payment will be determined as follows:
| • | the rights forming fractional shares will not be negotiable or transferable. Therefore, in accordance with the provisions of articles L. 228-6-1 and R. 228-12 of the French Commercial Code, when the number of Erytech shares to which a Pherecydes shareholder is entitled does not correspond to a whole number of Erytech shares, the shareholder will receive the number of Erytech shares immediately below, plus of a cash balance resulting from the price at which the Erytech shares corresponding to the fractional shares will have been sold by the financial intermediaries, within a period of thirty (30) days as from the latest of the dates of registration, in the account of the Pherecydes shareholders, of the whole number of Erytech shares allocated. |
| (b) | Date of dividend—date of trading and admission to listing |
The New Shares, that will be immediately assimilated to the existing shares of the Absorbing Company, will carry dividend rights as from their date of issue and will give the right to the benefit of all distributions of dividends or reserves decided as from this date. A double voting right will be attributed in accordance with the legal conditions to all fully paid-up shares for which proof of registration for at least two years is provided. The New Shares will all be negotiable as soon as the capital increase of the Absorbing Company remunerating the merger is completed, in accordance with article L. 228-10 of the French Commercial Code, and will immediately be the subject of an application for admission to trading on the regulated market of Euronext Paris.
36
It is specified that, on the Completion Date, the New Shares will not be registered with the Securities Exchange Commission or be the subject of an application for admission to trading on Nasdaq. Such registration shall be made within twelve (12) months after completion of the Merger.
| 3.2.3.4 | Accounting for the Merger |
Pursuant to the regulation of the French accounting standards authority (Autorité des normes comptables) n° 2014-03 of June 5, 2014, relating to the general chart of accounts as last amended by the ANC regulation n° 2022-01 of March 11, 2022, the contributions made in the context of the Merger shall be valued on the basis of their fair market value, determined according to the valuation methods described in Section 3.2.3.6 of this Exemption Document.
| (a) | Designation and value of assets contributed and liabilities assumed |
Designation and value of the contributed assets
The Merger of the Absorbed Company includes all the assets of this company for their real value as determined hereinafter (the net book value being given for information purposes only):
| Asset |
Net carrying amount (€) |
Fair market value (€) |
||||||
| Intangible assets |
9 071 772 | 18 087 000 | ||||||
| Goodwill |
0 | 1 017 000 | ||||||
| Other intangible fixed assets |
3 763 640 | 0 | ||||||
| Formation expenses |
0 | 0 | ||||||
| Development costs |
5 277 049 | 17 070 000 | ||||||
| Concessions, patents and similar assets |
31 083 | 0 | ||||||
| Advances on intangible fixed assets |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Tangible fixed assets |
559 184 | 559 184 | ||||||
| Land |
0 | 0 | ||||||
| Constructions |
0 | 0 | ||||||
| Industrial machinery |
135 094 | 135 094 | ||||||
| Other tangible fixed assets |
424 090 | 424 090 | ||||||
| Fixed assets in progress |
0 | 0 | ||||||
| Advances payments and accounts |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Financial fixed assets |
149 825 | 149 825 | ||||||
| Participation valued according to the equity method |
0 | 0 | ||||||
| Other equity interests |
||||||||
| Receivables from equity interests |
||||||||
| Other fixed equity securities |
||||||||
37
| Loans |
0 | 0 | ||||||
| Other financial assets |
0 | 0 | ||||||
| 25269 | 25269 | |||||||
| 0 | 0 | |||||||
| 124556 | 124556 | |||||||
|
|
|
|
|
|||||
| Total fixed assets |
9 780 782 | 18 796 009 | ||||||
|
|
|
|
|
|||||
| Inventory |
0 | 0 | ||||||
| Raw materials, supplies |
0 | 0 | ||||||
| Work in progress of goods |
0 | 0 | ||||||
| Intermediate and finished goods |
0 | 0 | ||||||
| Goods for resale |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Receivables |
2 367 790 | 2 367 790 | ||||||
| Advances payments on accounts on orders |
0 | 0 | ||||||
| Customers—Trade receivables and related accounts |
126 604 | 126 604 | ||||||
| Other receivables |
2 241 186 | 2 241 186 | ||||||
| Subscribed called-up unpaid capital |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Availability |
1 035 127 | 1 035 127 | ||||||
| Marketable securities |
0 | 0 | ||||||
| Availabilities |
1 035 127 | 1 035 127 | ||||||
|
|
|
|
|
|||||
| Prepaid expenses |
32 412 | 32 412 | ||||||
|
|
|
|
|
|||||
| Total current assets |
3 435 329 | 3 435 329 | ||||||
|
|
|
|
|
|||||
| Asset translation differences |
0 | 0 |
The fair market value of the assets included in the contribution therefore amounts to 22,231,338 euros.
Designation and value of liabilities supported
The Merger of the Absorbed Company is granted and accepted in consideration of the assumption by the Absorbing Company of all the liabilities of the Absorbed Company, namely:
| Liabilities |
Net carrying amount (€) |
Fair market value (€) |
||||||
| Provisions for liabilities and expenses |
0 | 0 | ||||||
| Provisions for liabilities |
0 | 0 | ||||||
| Provisions for expenses |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Financial debts |
3 070 146 | 3 070 146 | ||||||
|
|
|
|
|
|||||
38
| Convertible debenture loans |
0 | 0 | ||||||
| Other bond loans |
360 000 | 360 000 | ||||||
| Loans and debts from lending institutions |
2 178 264 | 2 178 264 | ||||||
| Other loans and financial debts |
531 882 | 531 882 | ||||||
|
|
|
|
|
|||||
| Operating liabilities |
2 383 806 | 2 383 806 | ||||||
| Advances payments on account received on orders in progress |
0 | 0 | ||||||
| Suppliers debts—Accounts payable and related accounts |
1 589 993 | 1 589 993 | ||||||
| Tax and social security liabilities |
791 278 | 791 278 | ||||||
| Fixed assets debts—Accounts payable on related accounts |
0 | 0 | ||||||
| Other debts |
2 535 | 2 535 | ||||||
|
|
|
|
|
|||||
| Deferred income |
240 000 | 240 000 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
5 693 952 | 5 693 952 | ||||||
|
|
|
|
|
|||||
| Unrealized exchange gains—Liabilities |
0 | 0 |
The total amount of the liabilities included in the contribution is therefore supported for an amount of 5,693,952 euros.
The net assets contributed by the Absorbed Company and remunerated by the Absorbing Company pursuant to article L. 236-3 II of the French Commercial Code therefore amount to 16,537,386 euros.
(b) Details of the calculation of the merger bonus
The difference between the net assets contributed by Pherecydes of 16,537,386 euros (i.e. net assets contributed of 16,479,810.89 euros after deduction of the treasury shares) and (i) the completion of the Contribution in Kind (1,894,132 euros) and (ii) the nominal amount of the capital increase of Erytech relating to the Merger (2,657,589.90 euros, accompanied by a total compensation of 0.42 euros) i.e. 12,099,841.12 euros, represents the merger premium and will be credited to an account “merger premium”.
| 3.2.3.5 | Conditional considerations |
There are no future events that condition the grant of additional securities to the shareholders of the Absorbed Company in connection with the Merger.
| 3.2.3.6 | Evaluation method |
The exchange ratio retained for the issuance of 26,575,893 newly issued ordinary shares by the Absorbing Company to the benefit of the shareholders of the Absorbed Company, was assessed according to a multi-criteria approach based on valuation methods usually used for the valuation of companies in the healthcare sector.
39
A summary description of these methods can be found in Annex 14.1 of the Merger Agreement (in Annex 1 of the Exemption Document).
| 3.2.3.7 | Break-up fees and penalties |
No break-up fee or any other penalty has been provided for in the Merger Agreement in the event that the Merger is not completed.
| 3.2.4 | Notifications and requests for authorization |
No third party notification or authorization request is required for the completion of the Merger, except for the approval of the extraordinary general meeting of the Absorbing Company and the Absorbed Company (please refer to Section 3.2.1. of the Exemption Document).
| 3.2.5 | Information on the financing structure of the Merger |
The implementation of the Merger does not require any specific structure for its financing that should be the subject of any presentation.
| 3.2.6 | Merger Timetable |
| Meeting of the Board of Directors of Erytech having approved the terms of the Merger Agreement | May 5, 2023 | |
| Meeting of the Board of Directors of Pherecydes having approved the terms of the Merger Agreement | May 5, 2023 | |
| Signature of the Merger Agreement | May 15, 2023 | |
| Availability of the reports of the Merger Auditor | May 15, 2023 | |
| Filing of the Merger Agreement with the Registries of the Commercial Court of Nantes and the Commercial Court of Lyon | May 16, 2023 | |
| Publication on the website of both companies of a notice relating to the Merger | May 17, 2023 | |
| General Meeting of Erytech called to approve the Merger and the change of the corporate name of Erytech | June 23, 2023 | |
| General Meeting meeting of Pherecydes called to approve the Merger and the change of the corporate name of Erytech | June 23, 2023 | |
| Meeting of the Board of Directors of Erytech acknowledging the final completion of the Merger and deciding on the subsequent capital increase, acting upon delegation of powers | June 23, 2023 | |
40
| Euronext notice relating to the issuance of the shares issued in consideration of the Merger | June 23, 2023 | |
| Settlement and admission to trading on Euronext Paris of the shares issued as consideration for the Merger | June 29, 2023 | |
| Registration with the SEC and listing on Nasdaq of the shares issued as consideration for the Merger | June 29, 2024 at the latest | |
| 3.3 | RISK FACTORS RELATED TO THE MERGER |
In addition to the risk factors relating to the Group and its business described in Chapter 2 “Facteurs de risque” of the Erytech 2022 Universal Registration Document , the investor is invited to take into account the following risk factors and other information contained in the Exemption Document before deciding to invest in Erytech shares. An investment in the shares of Erytech involves risks.
As of the date of the Exemption Document, Erytech has not identified any significant risks other than those described in the Erytech 2022 Universal Registration Document and the Exemption Document.
The risk factors described below are specific to the Merger and are presented from the most important to the least important. The risk factors related to the equity securities to be issued by Erytech in connection with the Merger are set forth in Section 4.1 “Risk Factors Related to Equity Securities” of the Exemption Document.
Risk related to the absence of variation of the number of new Erytech shares issued in consideration of the Merger with respect to the evolution of the share price of the Erytech and Pherecydes
As at the date of signature of the Merger Agreement, the exchange ratio was set at four (4) shares of Pherecydes for fifteen (15) shares of Erytech (as indicated in Section 3.5 of the Exemption Document). As no adjustment mechanism is provided for, the exchange ratio will remain unchanged and the Merger will be completed even if the market prices of the Erytech and/or Pherecydes shares would change after the execution of the Merger Agreement. However, the share prices of the Erytech and/or Pherecydes shares could vary or have varied significantly upwards or downwards on the Completion Date as compared to the date on which the exchange ratio of the Merger was set by the respective boards of directors of Erytech and Pherecydes on June 23, 2023. The Merger will therefore be completed at the initial exchange ratio even if the share prices of Erytech and/or Pherecydes would change significantly after the execution of the Merger Agreement.
41
Impact of the Merger on the share price of Erytech
The completion of the Merger could have an adverse effect on the share price of Erytech on the regulated market of Euronext Paris and on the trading price of the Erytech ADS on Nasdaq.
A significant and sustained decline in the share price of Erytech could have a material adverse effect on Erytech’s business, prospects, results, financial condition, reputation and development.
Risk related to the non-realization of certain conditions precedent
The completion of the Merger is subject to the satisfaction of several conditions precedent provided for in the Merger Agreement, namely:
| • | the delivery by the Merger Auditor of (i) a report on the value of the contributions and (ii) a report on the terms and conditions of the Merger confirming the fairness of the exchange ratio retained; |
| • | the approval by the extraordinary general meeting of the shareholders of (i) the Absorbed Company of the Merger, and of the resulting dissolution of the Absorbed Company, and (ii) the Absorbing Company, of the Merger, and of the corresponding capital increase of the Absorbing Company in consideration of the Merger; and |
| • | the approval by the extraordinary general meeting of the shareholders of the Absorbing Company of the resolutions relating to the appointment of the directors appointed by Pherecydes and the amendment of the articles of association of Erytech relating to the removal of the casting vote of the chairman of the board of directors. |
If these conditions are not fulfilled by midnight on July 31, 2023 at the latest, the Merger Agreement will be deemed to be null and void, without any indemnity on either side, unless waived by both Parties. The Merger would therefore not be completed and the development plans presented could not be realized immediately. Such events could have a material adverse effect on Erytech’s and Pherecydes’ business, results, financial condition and prospects.
Risk related to the integration of the activities of the two companies, to the costs related to integration and to the realization of synergies
The success of the Merger operation will be built as much in the preparation of the envisaged operation as in the integration process that will follow the legal completion of this operation. The two key points will be the ability of the new merged group to realize the synergies and to integrate the respective cultures. The realization of synergies will not be automatic. The new merged group may not have the tools and organization in place to identify the best practices of Erytech and Pherecydes. Moreover, a certain operational integration is necessary to obtain cost reductions, but it is not excluded that this integration could lead to a destruction of a certain value and competences necessary to its competitiveness. Indeed, the group thus formed might not have, or might not have sufficiently, evaluated, developed and worked on the compatibility of the organizations, the degree of transformation they can support and the management of this process. It could be difficult to reconcile the operational requirements and the strategic vision of the new entity. In addition, the Merger will lead to the relocation of Pherecydes’ activities currently located in Nantes to Erytech’s premises in Lyon, which may lead to the departure of certain key employees. The occurrence of one or more of these risks could have a significant adverse effect on the business of the new merged group, its results, its financial condition and its prospects.
42
Risk related to the need to retain management and key personnel following the Merger
The success of the new group formed as a result of the Merger will largely depend on its ability to retain and attract key executives and personnel of both companies. The inability of the merged group to retain, attract and retain key personnel could prevent it from achieving its objectives overall and thus have a material adverse effect on its business, results, financial condition and prospects.
Risk related to the U.S. regulatory compliance due to dual listing
As a result of the Merger, the shareholders of Pherecydes will become shareholders of a dual listed company. The listing of its shares, in the form of American Depositary Shares (ADS), in the United States on the Nasdaq Stock Market results in the Company being subject to a series of standards and regulations, including the reporting requirements of the Securities Exchange Act. All information concerning Pherecydes’ business will therefore be subject to regulatory disclosure requirements. Legal proceedings could be initiated by competitors or third parties on the basis of this information concerning the clinical programs developed by Pherecydes. If such claims are successful, the company’s business and operating results could be affected. Even if such legal actions do not result in a conviction against the Company, these proceedings, and the time and resources required to resolve them, may require the Company to use resources that should have been allocated to the company’s business. This would have a material adverse effect on the company’s business, financial condition, results, reputation or development.
| 3.4 | CONFLICTS OF INTERESTS |
The fund Auriga IV Bioseeds, a professional private equity fund represented by its management company Elaia Partners, a simplified joint stock company (société par actions simplifiée), whose registered office is located at 21 rue d’Uzès, 75002 Paris, France, registered with the Paris Trade and Companies Register under number 443 990 668, shareholder of Pherecydes, and the company Auriga Partners, a simplified joint stock company (société par actions simplifiée), whose registered office is located at 250 B rue du Faubourg Saint-Honoré, 75008 Paris, registered with the Paris Trade and Companies Register under number 419 156 351, shareholder of Erytech, are two separate and distinct entities, and invest from time to time in the same business sectors.
Elaia Partners and Auriga Partners have partnered in several joint investment projects.
Franck Lescure, partner at Elaia Partners and member of the board of directors of Pherecydes, was previously a partner at Auriga Partners and was a member of the board of directors of Erytech from December 2006 to January 2013.
| 3.5 | MERGER CONSIDERATION |
Please refer to paragraph 3.2.3 “Merger Consideration” of this Exemption Document.
43
| 4. | EQUITY SECURITIES ADMITTED TO TRADING ON A REGULATED MARKET FOR THE PURPOSE OF THE MERGER |
| 4.1 | RISK FACTORS RELATED TO EQUITY SECURITIES |
The risk factors described below are listed from most to least significant.
The present section of the Exemption Document identifies the risks specific to the issuance of new equity securities that may adversely affect Erytech and its equity securities.
Risk of an influx of Erytech shares for sale on the market
The shareholders of Pherecydes who will receive Erytech shares as consideration for the Merger may wish to sell their Erytech shares, which would result in an increase in the number of Erytech shares available for sale and affect the Erytech’s share price. The Company cannot predict the possible effects on the market price of the Company’s shares of potential sales of shares by its shareholders.
Risk related to the issuance of new shares in the context of the Merger resulting in a dilution of the existing shareholders of Erytech
The issuance of the new Erytech shares in the context of the Merger involves the issuance of 29,677,638 shares (of which 26,575,893 in respect of the Merger and 3,101,745 shares pursuant to the Contribution in Kind) to the benefit of the shareholders of Pherecydes, whereas the share capital of Erytech was composed of 31,018,553 shares before the Contribution in Kind. A shareholder holding 1% of the share capital of Erytech prior to the Contribution in Kind will therefore hold, upon completion of the Merger, 0.51% of the share capital of Erytech. The dilution that may result from the issuance of the new Erytech shares is described in Section 4.5 of this Exemption Document.
Risk related to the volatility and liquidity of the shares following the Merger
The stock markets have experienced significant fluctuations in recent years that have often been unrelated to the results of the companies whose shares are traded. Market fluctuations and health, geopolitical and economic conditions could increase the volatility of Erytech’s shares. Erytech’s share price could fluctuate significantly in response to various factors and events, including the Merger.
As an example, upon the announcement in August 2022 of the discontinuation of activities to submit an application for approval for Graspa in the treatment of patients with ALL, the share price fell by 25% compared to the average of the previous 20 share prices. In addition, in the event that liquidity for the market of shares admitted to trading on Euronext Paris is not sustained, the price of the ordinary share could be more volatile and it would become more difficult to buy or sell ordinary shares on the Euronext Paris market than to buy or sell ADS on the Nasdaq market. The dual listing of Erytech’s shares in two different currencies (euro and US dollar) opens the possibility of an arbitrage strategy between the two stock exchanges that could have an impact on the respective prices of the ADSs and the shares. It is however specified that the New Shares will neither be registered with the Securities and Exchange Commission (the “SEC”) nor be the subject of an application for admission to trading on Nasdaq. Such registration shall be made within twelve (12) months after completion of the Merger
44
| 4.2 | NET WORKING CAPITAL STATEMENT |
Erytech certifies that, in its opinion, Erytech’s net working capital is sufficient to meet its current obligations for a period of twelve (12) months as of the date of issuance of the Exemption Document and that, after taking into account the completion of the Merger, Erytech’s working capital will be sufficient to meet its current obligations for a period of twelve (12) months as of the date of issuance of the Exemption Document.
Descriptive and financial information about the material effects that the Merger will have on its financial statements is presented in Section 5.6 of the Exemption Document.
| 4.3 | INFORMATION CONCERNING THE EQUITY SECURITIES TO BE ADMITTED TO TRADING |
| 4.3.1 | Nature, class, amount and currency of issue of securities admitted to trading |
The 26,575,893 New Shares issued as consideration for the Merger, the admission of which to trading on Euronext Paris is requested, correspond to the maximum number of new shares with a nominal value of 0.10 euro. The dilution that may be generated as a result of the issuance of these new shares is presented in Section 4.5 of the Exemption Document.
The New Shares will carry current dividend rights and will be entitled to all distributions decided by the Absorbing Company as from their issue.
They will immediately be the subject of an application for admission to trading on the regulated market of Euronext in Paris (compartment C), on the same quotation line as the existing shares under the same ISIN FR0011471135, at the earliest on the date indicated in the indicative timetable set out in Section 3.2.6 of the Exemption Document.
It is specified that, on the Completion Date, the New Shares will not be registered with the SEC nor will they be the subject of an application for admission to trading on Nasdaq. Such registration shall be made within twelve (12) months after completion of the Merger.
The new shares will be denominated in euro.
| 4.3.2 | Resolutions, authorizations and approvals pursuant to which the securities will be created and/or issued |
The resolutions of the boards of directors of the Absorbing Company and the Absorbed Company dated May 5, 2023, authorizing the signature of the Merger Agreement are set forth in Annex 1 of this Exemption Document.
The General Meeting of the Absorbed Company will meet on June 23, 2023 to approve among others the resolutions set in Annex 3 of the Exemption Document, including the extraordinary resolution n° 5 and n°6 relating to the Merger.
The General Meeting of the Absorbing Company will meet on June 23, 2023 to approve among others the resolutions set out in Annex 3 of the Exemption Document, including the extraordinary resolutions n°17 to n°20 relating to the Merger.
45
| 4.3.3 | Restrictions on the free negotiability of securities |
No clause in the articles of association limits the free trading of the shares comprising the capital of the Absorbing Company.
| 4.3.4 | Takeover bids launched by third parties in respect of the Absorbing Company’s securities which have occurred during the last and current financial years |
No takeover bid was initiated for the shares of the Absorbing Company during the year ended December 31, 2022, nor during the current year.
| 4.4 | ADMISSION TO TRADING AND DEALING ARRANGEMENTS |
| 4.4.1 | Admission to trading |
The 26,575,893 New Shares issued in consideration of the Merger will be immediately admitted to trading on the regulated market of Euronext Paris (“Euronext Paris”) (compartment C), on the same quotation line as the existing shares under the same ISIN FR0011471135, it being specified that these shares will be admitted to trading at the earliest on June 29, 2023, as indicated in the indicative timetable set forth in Section 3.2.7 of this Exemption Document.
The new shares may be converted into ADS pursuant to the terms of the Deposit Agreement between Erytech and Bank of New York Mellon. Once converted, the ADS may be traded on Nasdaq in the same way as the existing ADS. The conversion into ADS may not be completed until 12 months after the closing of the Merger unless, following the completion of the Merger, the Company files a registration statement with the SEC to allow the registration of the ADS. As part of the Memorandum of Understanding, it was agreed that the company would use its best efforts to file a registration statement within 12 months following the Merger.
| 4.4.2 | Liquidity commitment, placement and underwriting |
As of the date of this Exemption Document, there are no firm commitments with entities that would like to act as intermediaries on the secondary markets and guarantee the liquidity of the new shares by acting as buyers and sellers.
| 4.4.3 | Lock-up agreement—abstention and/or retention undertaking |
As of the date of the Exemption Document, there are no lock-up agreements relating to the shares of the Absorbing Company.
| 4.5 | DILUTION |
For information purposes, the impact of the issuance of the New Shares in the context of the Merger on:
| • | the equity per share of the Absorbing Company (calculations based on the equity resulting from the IFRS accounts of the Absorbing Company as of December 31, 2022), |
| • | the participation in the capital of a shareholder of the Absorbing Company holding 1% of the share capital of the Absorbing Company, and |
| • | the number of voting rights of a shareholder of the Absorbing Company holding 1% of the voting rights of the Absorbing Company |
46
(x) prior to the completion of the Contribution in Kind and to the issuance of the New Shares (calculated on the basis of the number of shares composing the share capital of the Absorbing Company and of the number of voting rights as of April 30, 2023) and (y) subsequent to the completion of the Contribution in Kind and of the Merger is as follows:
| Share of consolidated equity per share (in €) |
Shareholder participation (in %) |
Shareholder voting rights (in %) |
||||||||||||||||||||||
| Non-diluted basis |
Diluted basis* |
Non-diluted basis |
Diluted basis* |
Non-diluted basis |
Diluted basis* |
|||||||||||||||||||
| Before the Contribution in Kind and the Merger |
0.757 | 0.757 | 1.00 | % | 1.00 | % | 1.00 | % | 1.00 | % | ||||||||||||||
| After the Contribution in Kind and the Merger |
0.387 | 0.383 | 0.51 | % | 0.51 | % | 0.52 | % | 0.52 | % | ||||||||||||||
(a) : after the Merger, the 684,273 shares to which the 123,950 warrants issued by the Absorbed Company prior to the present and which were, prior to the signature of the Memorandum of Understanding, exercisable and in the money, and the 58,523 ordinary shares allocated free of charge by the Absorbed Company prior to the present are taken into account in the calculation of the number of shares and voting rights of the Absorbing Company on a diluted basis.
(b) : after the Merger, the number of shares and voting rights of the Absorbing Company on a diluted basis does not take into account the shares to which all the dilutive instruments issued by the Absorbing Company give entitlement, as these were not in the money on the date of signature of the Memorandum of Understanding, it being specified that the warrants attached to the ABSAs are no longer exercisable as of the date of the Registration Document (the dilutive instruments of the Absorbing Company are described in Sections 3.1.2.1.3 and 3.3 of the Erytech 2022 Universal Registration Document).
Insofar as Pherecydes does not have double voting rights, its shareholders cannot benefit from this double voting right immediately after the completion of the Merger (even if they meet the two-year holding period at the time of the exchange) and will have to wait for the expiration of the holding period provided for in the articles of association of Erytech to be able to exercise the double voting right, since they cannot claim the benefit of article L. 225-124, paragraph 2 of the French Commercial Code.
47
| 4.5.1 | Structure chart |
The chart below shows the structure of the Absorbing Company after the completion of the Merger:
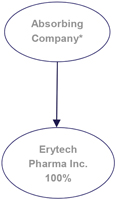
* The new corporate name will be voted at the extraordinary general meeting of June 23, 2023.
4.5.2 Concurrent offering of equity securities of the same class
None
| 4.6 | ADVISORS |
In the context of the Merger, ODDO BHF SCA acted as financial advisor.
| 5. | IMPACT OF THE MERGER ON THE ISSUER |
| 5.1 | STRATEGY AND OBJECTIVES |
The strategy and objectives of Erytech after completion of the Merger are described in Section 1.2 “Stratégie du Groupe” of the Erytech 2022 Universal Registration Document and in Section 3.1 of the Exemption Document.
| 5.2 | MATERIAL CONTRACTS |
It is specified that, as of May 5, 2023, Bpifrance has given its consent to the continuation within the Absorbing Company of the following contracts entered into by the Absorbed Company: (i) the State-guaranteed loan agreement dated April 27, 2021 for an amount of 2 million euros, as amended in January 2022 in order to benefit from an additional one-year deferral of repayment and a four-year repayment thereafter and (ii) the agreement dated February 22, 2022 for the financing of the PhagEcoli program for an amount of 1,687,800 euros, divided 60% in the form of a grant and 40% in the form of a reimbursable advance, to be paid at each milestone and conditional on the provision of certain elements by Pherecydes Pharma.
In addition, on May 5, 2023, (i) Société Générale has agreed to the continuation of the State-guaranteed loan entered into by the Absorbing Company on November 5, 2020, for a principal amount of 5 million euros and (ii) Bpifrance Financement has agreed to the continuation of the State-guaranteed loan entered into by the Absorbing Company on November 10, 2020, for a principal amount of 5 million euros.
As of the date of the Exemption Document, there are no other material contracts entered into within the Absorbing Company and/or within the Absorbed Company that would be impacted by the Merger.
48
| 5.3 | DISINVESTMENT |
None.
| 5.4 | CORPORATE GOVERNANCE |
| 5.4.1 | Composition of the Board of directors and general management |
Under the terms of the Memorandum of Understanding, and as announced in the press releases of Erytech and Pherecydes dated the February 15, 2023 (please refer to Sections 2.1.7 and 2.2.7), upon completion of the Merger, Mr. Jean-Paul Kress, Chairman of Erytech, will resign from his position and Mr. Didier Hoch, Chairman of Pherecydes, will become Chairman of the Board of Directors of Erytech.
Mr. Thibaut du Fayet, currently Chief Executive Officer of Pherecydes, will become Chief Executive Officer of Erytech, while Mr. Gil Beyen, currently Chief Executive Officer of Erytech, will become Vice-Chairman of the Board of Directors of Erytech (and will remain Executive Chairman of ERYTECH Pharma, Inc., Erytech’s U.S. subsidiary). Mr. Eric Soyer will remain Chief Operating Officer and Chief Financial Officer of Erytech. Mr. Jérôme Bailly will retain his position as Deputy Chief Executive Officer following the Merger.
On May 15, 2023, the Board of Directors of the Absorbing Company, which approved the final completion of the Contributions in Kind, also co-opted Mr. Didier Hoch and the company Go Capital, represented by Mrs. Leila Nicolas, as directors of the Absorbing Company. In addition, Mrs. Mélanie Rolli and Mr. Luc Dochez have resigned from their positions on the Board of Directors of Erytech. These co-optations are decided subject to ratification by the next annual general meeting of Erytech and for the remaining term of office of Mrs. Mélanie Rolli and Mr. Luc Dochez. Mr. Sven Andréasson will resign from his position as director upon completion of the Merger.
It is also specified that the composition of the committees of the board of directors will be modified to take into account new appointments and resignations.
49
Following the completion of the Merger, the members of the board of directors of Erytech will be the following:
| Last name, first name or corporate name, sex |
Mandate before the merger |
Independent Director |
Committees |
Shareholder | ||||
| Didier Hoch Man |
Pherecydes Pharma | No | Member and Chairman of the Strategy Committee Member of the Clinical Committee Member and Chairman of the Compensation and Nomination Committee |
Yes | ||||
| Gil Beyen Man |
Erytech Pharma | No | Member of the Audit Committee Member and Chairman of the Strategy Committee |
Yes | ||||
| Eric Leire Man |
Pherecydes Pharma | No | Member of the Audit Committee | No | ||||
| GO Capital SAS, represented by Madam Leila Nicolas Woman |
Pherecydes Pharma | No | Member of the Audit Committee | Yes | ||||
| Robert Sebbag Man |
Pherecydes Pharma | Yes | Member of the Clinical Committee | No | ||||
| Hilde Windels BV, represented by Madam Hilde Windels Woman |
Erytech Pharma | Yes | Member and Chairman of the Audit Committee Member and President of the Compensation and Nomination Committee |
Yes | ||||
| Martine Ortin George Woman |
Erytech Pharma | Yes | Member and President of the Clinical Committee | Yes | ||||
| Philippe Archinard Man |
Erytech Pharma | Yes | Member of the Clinical Committee Member and Chairman of the Compensation and Nomination Committee |
Yes | ||||
| Guy Rigaud (censor) Man |
Pherecydes Pharma | N/A | Member of the Audit Committee | Yes | ||||
Erytech and Pherecydes have agreed to establish a strategic steering committee that supervises the execution of the Memorandum of Understanding, and in particular the implementation of the Merger and the operational integration of the two companies. This strategic steering committee is composed of two representatives of Pherecydes, namely Mr. Didier Hoch and Mr. Thibault du Fayet, and two representatives of Erytech, namely Mr. Gil Beyen and Mr. Eric Soyer. It meets at least every two weeks since the signature of the Memorandum of Understanding and whenever an emergency situation requires it.
Didier Hoch, director
Didier Hoch was coopted as a director of Board of directors of Erytech during its meeting on May 15 2023. He served as Chairman of the former Supervisory board of Pherecydes and then, since January 2019, as Chairman of the Board of Directors. Didier Hoch is a physician and is also a director of a listed company, OSE Immunotherapeutics, and was previously director of DBV Technologies and Genticel. From 2011 to 2013 he was Chairman of Pevion Biotech and then from 2013 to 2018 of the Biovision Forum and of the startup gas pedal “Big Booster”. Didier Hoch was, from 2000 to 2010, Chairman of Sanofi-Pasteur- MSD, a joint-venture dedicated to vaccines, between Sanofi & Merck. Didier Hoch has also held various management positions at Rhône Poulenc Rorer, then Aventis (“VP Middle East -Africa”, Vice President Middle East & Africa). Former President of the Vaccine Europe association of vaccine manufacturers “Vaccine Europe” and Chairman of the Biotechnology Committee of LEEM.
50
Robert Sebbag, director
Robert Sebbag was a member of the former Supervisory board of Pherecydes and then, since December 2020, a member of the Board of Directors. Robert Sebbag is a physician and a member of the Paris Hospitals’ Infectious and Tropical Diseases Department at the Pitié Salpêtrière Hospital. He is also director and founding member of Action Contre la Faim and director of Fondation Mérieux. From 2006 to 2016 he was Vice President Access to Medicines at Sanofi. Previously he held various positions at Rhône Poulenc Santé, Fondation Elf, and Aventis-Pasteur. Former member of the Executive Committee of the CEO round table of the Gate Foundation and Director of Leem (Les entreprises du médicament).
Eric Leire, director
Eric Leire is physician and currently chairman and chief executive officer of Genflow Biosciences, Eric Leire is also a director of listed and non-listed biotechnology companies (Immunethep, BSIM Therapeutics and Inhatarget Therapeutics). Dr. Leire has served as CEO of several publicly and privately held US biotechnology companies. He has contributed to several IPOs of biotechnology companies on various markets (OMX.Nasdaq; OTC.QB and Nasdaq). He was a research partner at the Harvard AIDS Institute and partner at Biofund Venture Capital. He has an MD from the University of Grenoble and an MBA from HEC and Kellogg School of Management, Northwestern University. He is also the inventor of several patents in the pharmaceutical field.
GO Capital SAS, represented by Leila Nicolas, director
GO Capital SAS, represented by Leila Nicolas, was co-opted as a director by the Board of directors of Erytech during the meeting of the Board of directors on May 15, 2023 and served on the former Supervisory board and then as a director of the Board of directors between December 2017 and June 2023. Leila Nicolas started her career in 2004 as a product manager at Bayer Schering Pharma in the field of multiple sclerosis and oncology. She then joined the Biotechnology and Life Sciences world as strategic marketing manager of a start-up in Alsace (Polyplus-transfection). Leila Nicolas concluded several licensing agreements and acquired a strong sensitivity to intellectual property. Leila Nicolas then participated in the creation of a company that develops and sells environmental health analysis kits before joining Go Capital in late 2013.
Gil Beyen, Chief Executive Officer
Gil Beyen is Chief Executive Officer of Erytech since May 2013 and served as Erytech’s Chairman of the Board from May 2013 until June 2019. Gil Beyen has assisted Erytech since 2012 as a consultant and also served as Chairman of the Supervisory Board of Eyrtech from August 2012 to May 2013. Between 2000 and 2013, Mr. Beyen was Chief Executive Officer and Director of TiGenix (Euronext: TIG), a company he co-founded. He previously served as the head of the Life Sciences division of Arthur D. Little, an international management consulting firm, in Brussels. Mr. Beyen holds a MSc in Bioengineering from the University of Leuven (Belgium) and an M.B.A. from the University of Chicago.
51
Hilde Windels BV, represented by Hilde Windels, director
Hilde Windels has served as a member of the board of directors since 2014 and has served as the representative of Hilde Windels BV, the legal entity that holds this seat, since 2017. She has over 20 years of experience in corporate finance, capital markets and strategic initiatives. She currently serves as Chief Executive Officer of Antelope Dx BV and Director of MDx Health NV and Celyad SA. Prior to Antelope Dx BV, she was executive chairman of the board of directors and co-Chief Executive Officer of Mycartis NV, a private immune diagnostics company in Belgium and a spin-out of Biocartis Group NV. Ms. Windels initially joined Biocartis in August 2011 as its Chief Financial Officer, a position she held until September 2015 when she was appointed co-Chief Executive Officer, a position she held until early 2017, when she became interim Chief Executive Officer of Biocartis until September 2017. From early 2009 to mid-2011, she worked as an independent chief financial officer for several private biotechnology companies. Ms. Windels served as Chief Financial Officer of Devgen from 1999 to 2008 and as a member of its board of directors from 2001 to 2008. Ms. Windels holds a Masters in Economics from the University of Leuven (Belgium).
Martine Ortin George, director
Martine Ortin George has served as a member of the board of directors since 2014. Doctor of Medecine, Martine George has extensive experience in the United States in clinical research, medical affairs and regulatory matters with both large and small oncology companies. She currently serves as principal and senior executive consultant-life sciences for Global Development Inc. Dr. George held the position of Vice President in charge of Global Medical Affairs for Oncology at Pfizer from 2010 to 2015. Previously, Dr. George held the positions of Senior Vice President and Chief Medical Officer at GPC Biotech and Senior Vice President, Head of the Oncology Department at Johnson & Johnson. She is a qualified gynecologist and oncologist, trained in France and in Montreal. Dr. George began her career as Chief of Service at the Institut Gustave Roussy (France), was a visiting professor at the Memorial Sloan Kettering Cancer Center, and then held positions of increasing responsibility at Lederle Laboratories (a predecessor company to Pfizer Inc.), Sandoz (now a division of Novartis AG) and Rhône-Poulenc Rorer (today part of Sanofi).
Philippe Archinard, director
Philippe Archinard has served as a member of the board of directors since 2013 and was previously a member of the Supervisory Board from 2007 to 2013. Dr. Archinard is Deputy CEO, Innovation and Scientific Partnerships of the Institut Mérieux. He was General Manager, Chief Executive Officer and director of Transgene from 2004 to 2020, after spending 15 years in various senior positions with bioMérieux, including directing its U.S. subsidiary. Prior to joining Transgene, he served as chief executive officer of Innogenetics N.V., from 2000 to December 2004. Dr. Archinard is a chemical engineer, holds a Ph.D. in biochemistry from the University of Lyon (France), and completed Harvard Business School’s Program of Management PMD.
Guy Rigaud, observer
Guy Rigaud has 30 years of experience in private equity in more than 300 young regional companies (more than half of them in the technology sector). Founder and chairman of the Board of directors of Rhône Alpes Création from 1990 to 2012, Guy Rigaud has participated in five IPOs on Euronext in Paris and two on Nasdaq (in the context of industrial disposals). Guy Rigaud was a member of the Board of directors for 12 years of the April Group (insurance), a company listed on the regulated market of Euronext in Paris. Since 2012, Guy Rigaud is the founder and Managing Partner of a seed capital fund created with four family offices.
52
| 5.4.2 | Conflicts of interests |
To the best of Erytech’s knowledge, there is no conflict of interests between the duties performed on behalf of the Absorbing Company by the members of its administrative and management bodies and the private interests or other duties of these members.
| 5.4.3 | Restrictions on the disposal of securities held |
To the best of Erytech’s knowledge, there are no restrictions accepted by the members of the administrative and management bodies of Erytech regarding the transfer, within a certain period of time after the Merger, of the equity securities of the Absorbing Company that they hold.
| 5.4.4 | Compliance with the Middlenext code |
As of the date of the Exemption Document, the Absorbing Company complies with the Middlenext code, as detailed in Section 3.1.1.1 “La mise en oeuvre du Code Middlenext par la Société” of the Erytech 2022 Universal Registration Document. Upon completion of the Merger, the new entity intends to comply with the Middlenext code.
| 5.4.5 | Compensation and benefits |
In case of appointment of new corporate officers or renewal of the mandate of a corporate officer in the context of the definitive completion of the Merger, the compensation policy described in section 3.1.2.2 “Politique de rémunération des mandataires sociaux pour l’exercice 2023” of the Erytech 2022 Universal Registration Document will be applicable to them mutatis mutandis, subject to a possible decrease of the fixed, variable or exceptional remuneration decided by the board of directors and/or adjustments of certain other elements of remuneration according to the profile of the concerned persons.
The Absorbing Company intends to adjust the performance criteria upon completion of the Merger.
| 5.5 | PARTICIPATION |
| 5.5.1 | Shareholding |
The following table presents the capital structure of the Absorbing Company after completion of the Merger on the basis of the shareholding structure of the companies following the completion of the Contributions in Kind:
| Shareholders |
Number of Shares | % | ||||||
| Management |
25 830 | 0,04 | % | |||||
| Auriga Partners |
1 018 212 | 1,68 | % | |||||
| Recordati Orphan Drugs |
431 034 | 0,71 | % | |||||
| Directors |
10 303 | 0,02 | % | |||||
| Other shareholders |
42 655 | 0,07 | % | |||||
| Treasury shares |
2 500 | 0,00 | % | |||||
| BVF Partners L.P. |
97 338 | 0,16 | % | |||||
53
| Free float E |
29 390 681 | 48,42 | % | |||||
| Pherecydes’ shareholders contribution |
3 101 745 | 5,11 | % | |||||
| ELAIA Partners |
1 542 675 | 2,54 | % | |||||
| Go Capital |
1 058 535 | 1,74 | % | |||||
| Guy R Pool |
500 535 | 0,82 | % | |||||
|
|
|
|
|
|||||
| Erytech |
34 120 298 | 56,21 | % | |||||
|
|
|
|
|
|||||
| ELAIA Partners |
5 388 663 | 8,88 | % | |||||
| Go Capital |
3 697 533 | 6,09 | % | |||||
| Guy R Pool |
1 748 883 | 2,88 | % | |||||
| ACE |
5 192 115 | 8,55 | % | |||||
| Omnes Capital |
909 742 | 1,50 | % | |||||
| Management |
16 811 | 0,03 | % | |||||
| Free float P |
8 707 822 | 14,35 | % | |||||
| Participations Besançon |
914 321 | 1,51 | % | |||||
|
|
|
|
|
|||||
| Pherecydes |
26 575 890 | 43,79 | % | |||||
|
|
|
|
|
|||||
| Total |
60 696 188 | 100,00 | % | |||||
|
|
|
|
|
| 5.6 | NON AUDITED PRO FORMA FINANCIAL INFORMATION AS OF DECEMBER 31, 2022 |
INTRODUCTION
ERYTECH Pharma (“ERYTECH” or “the Company” or “the Absorbing Company”) and PHERECYDES Pharma (“PHERECYDES” or “the Absorbed Company”) announced the signing on February 15, 2023 of a Memorandum of Understanding (“MOU”) to enter into a strategic combination agreement (“Merger”) to create a global leader in phage therapy.
ERYTECH is a clinical-stage biotechnology company, founded in 2004, which develops innovative treatments, resulting from internal research and development programs, by encapsulating drugs in red blood cells.
PHERECYDES is a clinical-stage biotechnology company, founded in 2010, which develops innovative treatments, resulting from internal research and development programs, specialized in precision phage therapy for the treatment of resistant and/or complicated bacterial infections, using bacteriophages.
The strategic combination would leverage the complementary expertise and resources of the two companies to accelerate the development of phage therapy to fight antibiotic resistance, in particular through the PhagoDAIR Phase II study conducted by PHERECYDES, and its extension to other anti-infective and therapeutic areas with significant unmet medical needs.
The merger is expected to be completed by the end of the second quarter of 2023 with the vote of shareholders at the extraordinary general meetings of the two companies on June 23, 2023.
The proposed transaction aims to capitalize on ERYTECH’s financial resources and teams to both accelerate and expand PHERECYDES’ existing phage therapy development programs, launch new phage candidates, and potentially broaden the scope of application to new therapeutic modalities by leveraging both companies’ advanced technology platforms and expertise.
54
ERYTECH and PHERECYDES aim to merge their activities and relocate all teams to ERYTECH’s premises in Lyon, France, where they will benefit from a location within a major European cluster in the field of infectious diseases.
ERYTECH has received commitments from Auriga Partners (acting on behalf of Auriga Ventures III) and Recordati SpA, which together represent approximately 4.67% of the share capital and 8.91% of the voting rights of ERYTECH, to vote in favor of the resolutions related to the transaction at the ERYTECH EGM. Similarly, PHERECYDES has received commitments from Elaia Partners (acting on behalf of Auriga IV Bioseeds), Go Capital (acting on behalf of Ouest Ventures III) and the pool of shareholders represented by Mr. Guy Rigaud, who together represent approximately 41.5% of the share capital and voting rights of PHERECYDES, to contribute their PHERECYDES shares to ERYTECH in exchange for ERYTECH shares and to vote in favor of the resolutions related to the transaction at the ERYTECH EGM.
The completion of the merger is subject to the following conditions:
| • | the delivery by the Merger Auditor of (i) a report on the value of the contributions and (ii) a report on the terms and conditions of the Merger confirming the fairness of the exchange ratio retained; |
| • | the approval by the general shareholders’ meeting of the Absorbed Company of the Merger and the resulting dissolution of the Absorbed Company; |
| • | the approval by the general shareholders’ meeting of the Absorbing Company of the Merger as well as of the capital increase allowing the remuneration of the PHERECYDES contributions (as set forth in Article 14.3 of the merger agreement); and |
| • | the approval by the general shareholders’ meeting of the Absorbing Company of the resolutions relating to the appointment of the directors designated by PHERECYDES and the modification of the by-laws of Erytech relating to the suppression of the casting vote of the chairman of the board of directors. |
As of the date of this document, the Company considers that the conditions listed above are highly probable.
The date of final completion of the Merger and of the capital increase of the Absorbing Company will be the date of completion of the last of the conditions referred to above. The deadline for the fulfillment of the conditions is set at midnight on July 31, 2023 at the latest, after which the merger agreement will lapse.
MAIN TERMS OF THE MERGER PROJECT
The transaction is structured as a merger by absorption of PHERECYDES into ERYTECH, whereby PHERECYDES shareholders will receive newly issued shares of ERYTECH common stock in consideration for the contribution of PHERECYDES’ assets and liabilities. Upon completion of the merger, all of PHERECYDES’ assets and liabilities will be transferred to ERYTECH and PHERECYDES will be dissolved.
On February 15, 2023, ERYTECH and PHERECYDES have entered into a memorandum of understanding and plan to sign a definitive merger agreement to merge the companies in a full share exchange transaction. PHERECYDES shareholders will receive 15 new ERYTECH shares for 4 PHERECYDES shares. At the end of the transaction, PHERECYDES shareholders will hold approximately 49.5% of the share capital and voting rights of ERYTECH.
55
The terms and conditions of the Merger are established on the basis of the annual accounts of the financial year ending on December 31, 2022 of both companies. No control situation existed in 2022 between the initiating Company (ERYTECH) and the target Company (PHERECYDES). The two companies were totally independent and did not have any business relationship. After examining all the facts and circumstances related to the transaction as well as the provisions of the memorandum of understanding and the merger agreement, the management of ERYTECH and PHERECYDES determined that ERYTECH would be the acquirer for accounting purposes within the meaning of IFRS 3 “Business combinations”. The absorbing company is ERYTECH, the absorbed company is PHERECYDES.
As this is a conventional merger, involving entities under separate control, as defined by IFRS 3, the value of the assets contributed by the absorbed Company in the context of the acquisition of PHERECYDES by ERYTECH will be the real value. The terms of the contribution in kind of PHERECYDES shares to ERYTECH are as follows:
| • | The proposed merger provides for the conclusion of a contribution in-kind agreement, prior to the completion of the merger, between the shareholders of the absorbed company: Auriga IV Bioseeds (represented by Elaia Partners), Ouest Ventures III (represented by Go Capital) and the shareholder pool represented by Mr. Guy Rigaud, on the one hand, and the merging company ERYTECH, on the other. |
| • | The shareholders of the Absorbed Company have agreed to contribute, before the Completion Date, 827,132 PHERECYDES shares to the Absorbing Company in consideration of newly issued ERYTECH shares, according to the same exchange ratio as the Merger and to (ii) participate in the general shareholders’ meeting of the Absorbing Company convened to approve the Merger Proposal and to vote in favor of the Merger Proposal at such meeting. |
| • | The value of the contributed PHERECYDES shares has been set in the contribution agreement at €1,894,132, i.e. €2.29 per share, by reference to the spot market price of PHERECYDES on January 19, 2023, corresponding to the date of signature of the letter of intent (“LOI”) and of the determination of the parity in the context of the Merger. In order to remunerate this contribution, ERYTECH will proceed to a capital increase by issuing 3,101,745 new shares. This capital increase of ERYTECH has been included in the pro forma consolidated statement of financial position. |
| • | Realization at the date of the merger of a capital increase of ERYTECH for a total amount of €25.3M, in remuneration of the contribution of 7,086,905 PHERECYDES shares held by the shareholders of the absorbed company. This capital increase results in the issue of 26,575,894 new shares with a nominal value of 10 cents (€0.1). |
CAPITAL INCREASE CARRIED OUT BY PHERECYDES PRIOR TO THE MERGER
On February 17, 2023, PHERECYDES completed a capital increase for a total amount of €1.5 million, fully subscribed by the Company’s historical shareholders: Auriga IV Bioseeds (represented by Elaia Partners), Ouest Ventures III (represented by Go Capital) and the pool of shareholders represented by Mr. Guy Rigaud, for a subscription amount of €747K, €512K and €241K respectively. The realization of this capital increase allows PHERECYDES to fulfill one of the conditions of the merger, i.e. to finance the cash needs of PHERECYDES until the completion of the merger.
56
The €1.5M capital increase will result in the issuance of 717,702 new shares with a par value of one euro (€1) each, based on a subscription price of €2.09 per share. The subscription price represents a discount of 21.4% on the closing price on February 17, 2023.
The share capital of PHERECYDES after the increase amounts to €7,939,179, divided into 7,939,179 shares.
BASIS OF PREPARATION OF THE UNAUDITED PRO FORMA FINANCIAL INFORMATION
The Pro Forma Financial Information is defined as the unaudited pro forma consolidated financial information of the ERYTECH Group after taking into account the effects of the merger by absorption of PHERECYDES, which is expected to be completed by the end of the second quarter of 2023, and of the capital increase completed in February 2023 by PHERECYDES (together, the “Transactions”). The Pro Forma Financial Information has been prepared as if the Transactions had become effective as of January 1, 2022 for the pro forma consolidated statement of net income and as of December 31, 2022 for the pro forma consolidated statement of financial position, and includes a pro forma consolidated statement of net income covering the period from January 1, 2022 to December 31, 2022 and a pro forma consolidated statement of financial position as of December 31, 2022.
The Pro Forma Financial Information is prepared in accordance with the accounting principles used for the preparation of ERYTECH’s audited historical consolidated financial statements in accordance with International Financial Reporting Standards (“IFRS”).
The Pro Forma Financial Information is prepared in accordance with the provisions of the Delegated Regulation (EU) 2021-528 on exemption documents, the ESMA guidance on prospectuses of July 2020 and the AMF recommendations on pro forma financial information included in the guide for the preparation of universal registration documents of January 2022.
The Pro Forma Financial Information is intended to present the anticipated effects of the Transactions on the historical financial statements of the Erytech Group for the year ending December 31, 2022. It is not necessarily indicative of the financial position and performance that would have occurred had the Transactions actually occurred on January 1, 2022.
The Pro Forma Financial Information consists of the unaudited pro forma financial statements as of December 31, 2022 (the “Pro Forma Consolidated Financial Statements”), i) Pro Forma Consolidated Statement of Net Income and ii) Pro Forma Consolidated Statement of Financial Position, and explanatory notes.
The Pro Forma Financial Information of the Erytech Group has been prepared on the basis of the assumptions summarized below and should be read in conjunction with the following documents:
| • | The historical consolidated financial statements of the ERYTECH Group for the year ended December 31, 2022 prepared in accordance with IFRS as adopted by the European Union (EU) and audited by the auditors KPMG and RSM; and |
| • | The financial statements included in the Annual Financial Report of PHERECYDES for the year ended December 31, 2022, prepared in accordance with French GAAP and audited by PricewaterhouseCoopers Audit. |
The pro forma adjustments taken into account in preparing the Pro Forma Financial Information are limited to those directly attributable to the Transactions and which can be supported by facts, and are based on assumptions that ERYTECH considers reasonable as of the date of this document and in the context of the Transactions.
57
The Pro Forma Financial Information does not include any consequences of the expected synergies and does not provide any indication of the future results and condition of the business or of the reorganization and integration costs that may be incurred as a result of the Transactions.
No reciprocal transactions have been identified in the historical financial data of ERYTECH and PHERECYDES.
The pro forma adjustments presented below are based on information available to date and on assumptions and estimates that ERYTECH considers reasonable.
The Pro Forma Financial Information is presented in thousands of euros.
58
UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL STATEMENTS OF THE ERYTECH GROUP AS OF DECEMBER 31, 2022
Pro forma consolidated statement of net income as of December 31, 2022 - Unaudited
| (in K€) | ERYTECH Published |
PHERECYDES in |
IFRS transition adjustments |
Pro forma adjustments |
Transaction costs |
Pro forma accounts |
||||||||||||||||||
| IFRS | ERYTECH | PHERECYDES | 31/12/2022 | |||||||||||||||||||||
|
|
presentation |
|
|
|
|
|||||||||||||||||||
| Notes |
1 | 2 | 3 | 4 | 8 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Revenue |
— | — | — | — | — | |||||||||||||||||||
| Other income |
30,998 | 3,763 | (1,751 | ) | — | — | 33,010 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating IncomeOperating income |
30,998 | 3,763 | (1,751 | ) | — | — | 33,010 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Research and development costs |
(19,907 | ) | (6,831 | ) | 72 | (157 | ) | — | (26,823 | ) | ||||||||||||||
| Selling, general and administrative expenses |
(13,887 | ) | (3,111 | ) | (74 | ) | — | (3,073 | ) | (20,144 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating expenses |
(33,794 | ) | (9,942 | ) | (2 | ) | (157 | ) | (3,073 | ) | (46,968 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Operating loss |
(2,796 | ) | (6,179 | ) | (1,753 | ) | (157 | ) | (3,073 | ) | (13,958 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Financial income |
4,453 | 1 | — | — | — | 4,454 | ||||||||||||||||||
| Financial expenses |
(1,364 | ) | (65 | ) | (57 | ) | — | — | (1,485 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Financial income (loss) |
3,089 | (64 | ) | (57 | ) | — | — | 2,969 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income tax (*) |
(521 | ) | 1,388 | (1,388 | ) | — | — | (521 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
(228 | ) | (4,854 | ) | (3,198 | ) | (157 | ) | (3,073 | ) | (11,510 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (*) | the tax impacts of IFRS transition adjustments and pro forma adjustments are described in note 5. |
59
Pro Forma Consolidated Statement of Financial Position as of December 31, 2022—Unaudited: ASSETS
| (in K€) | ERYTECH Published IFRS |
PHERECYDES in ERYTECH presentation |
IFRS transition adjustments PHERECYDES |
Pro forma adjustments |
Capital increase PHERECYDES |
Business combination |
Transaction costs |
Pro forma accounts |
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
31/12/2022 | |||||||||||||||||||||||||
| Notes |
1 | 2 | 3 | 4 | 5 | 7 | 8 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| ASSETS |
||||||||||||||||||||||||||||||||
| Non-current assets |
||||||||||||||||||||||||||||||||
| Intangible assets |
5 | 9,072 | (9,041 | ) | 36 | |||||||||||||||||||||||||||
| Goodwill |
— | — | — | 29,197 | 29,197 | |||||||||||||||||||||||||||
| Property, plant and equipment |
393 | 559 | — | (148 | ) | 804 | ||||||||||||||||||||||||||
| Right of use |
2,584 | — | 1,438 | (846 | ) | 3,175 | ||||||||||||||||||||||||||
| Other non-current assets |
195 | 150 | (25 | ) | — | 319 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total non-current assets |
3,177 | 9,781 | (7,628 | ) | (995 | ) | — | 29,197 | 33 532 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Current assets |
||||||||||||||||||||||||||||||||
| Trade and other receivables |
76 | 127 | — | 202 | ||||||||||||||||||||||||||||
| Other current assets |
3,769 | 2,273 | — | 6,042 | ||||||||||||||||||||||||||||
| Cash and cash equivalents |
38,789 | 1,035 | — | 1,500 | 41,324 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Total current assets |
42,634 | 3,434 | — | — | 1,500 | — | 47,569 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| TOTAL ASSETS |
45,811 | 13,215 | (7,628 | ) | (995 | ) | 1,500 | 29,197 | 81,100 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
60
Pro forma consolidated statement of financial position as of December 31, 2022—Unaudited: LIABILITIES & SHAREHOLDERS’ EQUITY
| (in K€) | ERYTECH Published IFRS |
Pherecydes in ERYTECH presentation |
IFRS transition adjustments PHERECYDES |
Pro forma adjustments |
Capital increase PHERECYDES |
Buisness combination |
Transaction costs |
Pro forma accounts |
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
31/12/2022 | |||||||||||||||||||||||||
| Notes |
1 | 2 | 3 | 4 | 5 | 7 | 8 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| LIABILITIES AND EQUITY |
||||||||||||||||||||||||||||||||
| Shareholders’ equity |
||||||||||||||||||||||||||||||||
| Share capital |
3 102 | 7,221 | — | 718 | (4,971 | ) | 6,070 | |||||||||||||||||||||||||
| Premiums related to share capital |
48,975 | 8,904 | — | 782 | 15,629 | 74,290 | ||||||||||||||||||||||||||
| Reserves |
(29,765 | ) | (3,749 | ) | (5,923 | ) | 22 | 9,649 | (29,765 | ) | ||||||||||||||||||||||
| Translation reserves |
1,402 | — | — | — | 1,402 | |||||||||||||||||||||||||||
| Net loss for the period |
(227 | ) | (4,854 | ) | (3,198 | ) | (158 | ) | 8,210 | (3,073 | ) | (3,301 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total shareholders’ equity |
23,486 | 7,522 | (9,121 | ) | (136 | ) | 1,500 | 28,517 | (3,073 | ) | 48,696 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Non-current liabilities |
||||||||||||||||||||||||||||||||
| Provisions—non-current portion |
419 | — | 75 | (33 | ) | 461 | ||||||||||||||||||||||||||
| Financial liabilities—non-current portion |
7,547 | 2,540 | — | 10,086 | ||||||||||||||||||||||||||||
| Lease liabilities—non-current portion |
2,680 | — | 1,170 | (856 | ) | 2,994 | ||||||||||||||||||||||||||
| Total non-current liabilities |
10,646 | 2,540 | 1,245 | (889 | ) | — | — | — | 13,541 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Current liabilities |
||||||||||||||||||||||||||||||||
| Provisions—current portion |
314 | — | — | 314 | ||||||||||||||||||||||||||||
| Financial liabilities—current portion |
2,565 | 531 | (59 | ) | 3,037 | |||||||||||||||||||||||||||
| Lease liabilities—current portion |
775 | — | 306 | 30 | 1,112 | |||||||||||||||||||||||||||
| Trade and other payables |
5,115 | 1,590 | — | 680 | 3,073 | 10,459 | ||||||||||||||||||||||||||
| Current income tax liabilities |
512 | — | — | 512 | ||||||||||||||||||||||||||||
| Other current liabilities |
2,397 | 1,033 | — | 3,429 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| Total current liabilities |
11,678 | 3,153 | 248 | 30 | — | 680 | 3,073 | 18,863 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| TOTAL LIABILITIES AND EQUITY |
45,811 | 13,215 | (7,628 | ) | (995 | ) | 1,500 | 29,197 | — | 81,100 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (*) | Financial liabilities due in more than one year include 5 million euros of the PGE BPI ERYTECH loan and 2 million euros of the PGE BPI PHERECYDES loan, the contract of which provides for an early repayment clause in the event of a change of control. The post-merger loan was granted by BPI. |
61
EXPLANATORY NOTES TO THE PRO FORMA FINANCIAL INFORMATION
Note 1: ERYTECH Published IFRS
The historical data in this column is derived from the audited IFRS consolidated financial statements of the Erytech Group as published in the Universal Registration Document for the year ending December 31, 2022 and audited by KPMG and RSM.
Note 2: PHERECYDES in ERYTECH Presentation
The data in this column is taken from the individual financial statements of PHERECYDES prepared in accordance with French GAAP and audited by PricewaterhouseCoopers Audit as published in the annual financial report for the year ended December 31, 2022. Presentation adjustments have been applied to align with the presentation applied by the ERYTECH group.
A detailed reconciliation of the income statement published by PHERECYDES to the ERYTECH presentation is presented on the following page.
The transition from the published PHERECYDES income statement to the ERYTECH presentation is as follows:
| ERYTECH Presentation | ||||||||||||||||||||||||||||||||||||
| (in K€) |
Published PHERECYDES historical data |
Other income |
Research and development costs |
Selling, general and administrative expenses |
Operating expenses |
Operating loss |
Financial income (loss) |
Income tax |
Net income |
|||||||||||||||||||||||||||
| OPERATING INCOME |
3 167 | 3 167 | — | 3,167 | 3,167 | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Operating expenses |
— | |||||||||||||||||||||||||||||||||||
| Other purchases and external expenses |
5,961 | 4,396 | 1,565 | 5,961 | 5,961 | — | 5,961 | |||||||||||||||||||||||||||||
| Taxes and similar payments |
79 | 42 | 38 | 79 | 79 | — | 79 | |||||||||||||||||||||||||||||
| Wages and salaries |
2,331 | 1,398 | 932 | 2,331 | 2,331 | — | 2,331 | |||||||||||||||||||||||||||||
| Social security expenses |
991 | 594 | 396 | 991 | 991 | — | 991 | |||||||||||||||||||||||||||||
| Depreciation and amortization on fixed assets |
444 | 400 | 44 | 444 | 444 | — | 444 | |||||||||||||||||||||||||||||
| Other expenses |
51 | 0 | 51 | 51 | 51 | — | 51 | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| OPERATING EXPENSES |
9,857 | — | 6,831 | 3,026 | 9,857 | 9,857 | — | — | 9,857 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| OPERATING RESULT |
(6,690 | ) | 3,167 | (6,831 | ) | (3,026 | ) | (9,857 | ) | (6,690 | ) | — | — | (6,690 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Financial income |
||||||||||||||||||||||||||||||||||||
| Other interest and similar income |
1 | — | — | 1 | 1 | |||||||||||||||||||||||||||||||
| Foreign Currency Gains |
0 | — | — | 0 | 0 | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
| FINANCIAL INCOME |
1 | — | — | — | — | — | 1 | — | 1 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Financial expenses |
||||||||||||||||||||||||||||||||||||
| Financial amortization, depreciation and provisions |
12 | — | — | 12 | 12 | |||||||||||||||||||||||||||||||
| Interest and similar expenses |
53 | — | — | 53 | 53 | |||||||||||||||||||||||||||||||
| Foreign currency losses |
0 | — | — | 0 | 0 | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
| FINANCIAL EXPENSES |
65 | — | — | — | — | — | 65 | — | 65 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| FINANCIAL RESULT |
(64 | ) | — | — | — | — | — | (64 | ) | — | (64 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| CURRENT RESULT BEFORE TAX |
(6,754 | ) | 3,167 | (6,831 | ) | (3,026 | ) | (9,857 | ) | (6,690 | ) | (64 | ) | — | (6,754 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
62
| Extraordinary income |
||||||||||||||||||||||||||||||||||||
| On management operations |
240 | 240 | — | 240 | — | 240 | ||||||||||||||||||||||||||||||
| On capital transactions |
356 | 356 | — | 356 | — | 356 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| EXTRAORDINARY INCOME |
596 | 596 | — | — | — | 596 | — | — | 596 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Extraordinary expenses |
— | |||||||||||||||||||||||||||||||||||
| On capital transactions |
85 | 85 | 85 | 85 | — | 85 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| EXTRAORDINARY EXPENSES |
85 | — | — | 85 | 85 | 85 | — | — | 85 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| EXTRAORDINARY RESULT |
511 | 596 | — | (85 | ) | (85 | ) | 511 | — | — | 511 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Income taxes (*) |
1,388 | — | — | 1,388 | 1,388 | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
| PROFIT OR LOSS |
(4,854 | ) | 3,763 | (6,831 | ) | (3,111 | ) | (9,942 | ) | (6,179 | ) | (64 | ) | 1,388 | (4,854 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Corresponds with line-by-line reconciliation of the pro forma consolidated statement of net income as of December 31, 2022—Unaudited (Page 5)
|
| |||||||||||||||||||||||||||||||||||
| PHERECYDES in ERYTECH presentation |
3,763 | (6,831 | ) | (3,111 | ) | (9,942 | ) | (6,179 | ) | (64 | ) | 1,388 | (4,854 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| (*) | Research tax credit presented as a positive amount in the application of the sign convention used in the ERYTECH accounts. |
The following presentation changes have been made to restore consistency with the ERYTECH presentation:
| • | The published income statement of PHERECYDES has undergone a change in the presentation of expenses, by nature to a presentation by function, more consistent with the presentation of the ERYTECH income statement. |
| • | Operating expenses totaling €9,857,000 were allocated between research for €6,831,000 and development and selling , general and administrative expenses for€3,026,000. |
| • | The extraordinary result of €511K includes subsidies for €596K presented in other income and €85K of expenses related to the loss of value on treasury shares presented in selling, general and administrative expenses. |
In the published balance sheet of PHERECYDES, the items “conditional advances” (presented under “other equity” in the annual financial statement) for €532K, “loans and debts to credit institutions” for €360K and “loans and miscellaneous financial debts” for €2,169K, i.e. a total of €3,071K, are broken down into non-current financial for €2,540K and current financial debts for €531K.
This standardization exercise is carried out on a preliminary basis, based on the information available at this stage, and may therefore be subject to subsequent adjustments after the merger has been completed.
Note 3: IFRS transition adjustments PHERECYDES
This column presents the transition adjustments from French GAAP to IFRS identified in relation to the PHERECYDES individual financial statements on the basis of analyses conducted to date. Details of the adjustments identified as of the date of the Pro Forma Financial Information are summarized in the tables below.
Note 3: Details of the IFRS PHERECYDES adjustments for the pro forma consolidated statement of net income as of December 31, 2022—Unaudited
63
| (in K€) | IAS 19 | IAS 20 | IAS 38 | IAS 32 | IFRS 2 | IFRS 9 | IFRS 16 |
IFRS adjustments |
||||||||||||||||||||||||
| Revenues |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Other income |
— | 1,388 | (3,155 | ) | (0 | ) | — | 17 | — | (1,751 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Operating income |
— | 1,388 | (3,155 | ) | (0 | ) | — | 17 | — | (1,751 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Research and development |
15 | — | 325 | — | (306 | ) | (3 | ) | 40 | 72 | ||||||||||||||||||||||
| Selling, general and administrative |
(13 | ) | — | — | 86 | (146 | ) | — | — | (74 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Operating expenses |
2 | — | 325 | 86 | (451 | ) | (3 | ) | 40 | (2 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Operating income (loss) |
2 | 1,388 | (2,831 | ) | 85 | (451 | ) | 14 | 40 | (1,753 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Financial income |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Financial expenses |
— | — | — | 12 | — | (15 | ) | (55 | ) | (57 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Financial income (loss) |
— | — | — | 12 | — | (15 | ) | (55 | ) | (57 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Income tax |
— | (1,388 | ) | — | — | — | — | — | (1,388 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net income (loss) |
2 | — | (2,831 | ) | 97 | (451 | ) | (1 | ) | (14 | ) | (3,198 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
Note 3: Details of IFRS PHERECYDES adjustments in the pro forma consolidated statement of financial position as of December 31, 2022—Unaudited—ASSETS
| (in K€) | IAS 19 | IFRS 2 | IAS 32 | IFRS 16 | IAS 38 | IFRS 9 | IFRS adjustments |
|||||||||||||||||||||
| ASSETS |
||||||||||||||||||||||||||||
| Non-current assets |
||||||||||||||||||||||||||||
| Intangible assets |
— | — | — | — | (9,041 | ) | — | (9,041 | ) | |||||||||||||||||||
| Goodwill |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Property, plant and equipment |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Right of use |
— | — | — | 1,438 | — | — | 1,438 | |||||||||||||||||||||
| Other non-current assets |
— | — | (25 | ) | — | — | — | (25 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total non-current assets |
— | — | (25 | ) | 1,438 | (9,041 | ) | — | (7,628 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Current assets |
||||||||||||||||||||||||||||
| Inventories |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Trade and other receivables |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Other current assets |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Cash and cash equivalents |
— | — | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total current assets |
— | — | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| TOTAL ASSETS |
— | — | (25 | ) | 1,438 | (9,041 | ) | — | (7,628 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
64
Note 3: Details of IFRS PHERECYDES adjustments in the pro forma consolidated statement of financial position as of December 31, 2022 - Unaudited - LIABILITIES AND SHAREHOLDERS’ EQUITY
| (in K€) | IAS 19 | IFRS 2 | IAS 32 | IFRS 16 | IAS 38 | IFRS 9 | IFRS adjustments |
|||||||||||||||||||||
| LIABILITIES AND EQUITY |
||||||||||||||||||||||||||||
| Shareholders’ equity |
||||||||||||||||||||||||||||
| Share capital |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Premiums related to share capital |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Reserves |
(77 | ) | 451 | (122 | ) | (25 | ) | (6,210 | ) | 59 | (5,923 | ) | ||||||||||||||||
| Translation reserves |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Net income (loss) |
2 | (451 | ) | 97 | (14 | ) | (2,831 | ) | (1 | ) | (3,198 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total shareholders’ equity |
(75 | ) | — | (25 | ) | (39 | ) | (9,041 | ) | 59 | (9121 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Non-current liabilities |
||||||||||||||||||||||||||||
| Provisions - non-current portion |
75 | — | — | — | — | — | 75 | |||||||||||||||||||||
| Financial liabilities - non-current portion |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Derivative liabilities - non-current portion |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Lease liabilities - non-current portion |
— | — | — | 1,170 | — | — | 1,170 | |||||||||||||||||||||
| Deferred tax liability |
— | — | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total non-current liabilities |
75 | — | — | 1,170 | — | — | 1,245 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Current liabilities |
||||||||||||||||||||||||||||
| Provisions - current portion |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Financial liabilities - current portion |
— | — | — | — | — | (59 | ) | (59 | ) | |||||||||||||||||||
| Derivative liabilities - current portion |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Lease liabilities - current portion |
— | — | — | 306 | — | — | 306 | |||||||||||||||||||||
| Trade and other payables |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Current income tax liabilities |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Other current liabilities |
— | — | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total current liabilities |
— | — | — | 306 | — | (59 | ) | 247 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| TOTAL LIABILITIES AND EQUITY |
(25 | ) | 1,438 | (9,041 | ) | (7,628 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Adjustment of grants (IAS 20)
PHERECYDES recognized a tax income of €1,388K on December 31, 2022. This tax income relates to the research tax credit, which according to IAS 20 has been reclassified as other operating income, in accordance with the accounting principles applied by Erytech.
Adjustment of the pension liability (IAS 19)
Taking into account the assumptions made by PHERECYDES as of December 31, 2022, the liability for pension commitments under IFRS is €75,000.
65
Adjustment of share-based payments (IFRS 2)
The assumptions used by PHERECYDES to calculate the founder’s share warrants (otherwise known as bons de souscription de parts de créateurs d’entreprise, or BSPCE) and free share plans in the process of vesting on December 31, 2022, have led to the recognition of an expense of €451K against reserves. The charge in the pro forma consolidated statement of net income was broken down between research and development costs and selling, general and administrative expenses for €306K and €146K respectively.
Adjustment of lease obligations (IFRS 16)
The analysis of PHERECYDES’ lease contracts led to the recognition of a right of use with a net value of €1,438K, a lease liability of €1,476K, a decrease in research and development costs of €40K and a financial expense of €55K.
Adjustment of intangible assets (IAS 38)
Research and development costs for PHERECYDES’ programs in progress that have not received marketing approval from the health authorities, recorded in the parent company financial statements as intangible assets as of December 31, 2022 for a gross value of €10,582K and a net value of €9,041K, have been neutralized under IFRS, resulting in an amount of €6,210K in reserves and €2,831K on the income statement for the year. The impact on the income statement consists of the neutralization of i) the capitalized production (other income) related to these programs for €3,155 K€ and ii) the amortization expense related to capitalized development costs for €325K (presented in research and development expenses).
Note 4: Pro forma adjustments
The adjustments recorded in connection with the transition from French GAAP to IFRS of the PHERECYDES individual financial statements have been subject to consistency adjustments in the context of the merger. These pro forma adjustments also include the effect of fair value measurements of rights of use, lease liabilities and pension obligations, taking into account the changes announced in connection with the merger.
Details of the adjustments by standard are summarized in the tables below:
Note 4: Details of pro forma PHERECYDES adjustments to the Pro forma Consolidated Statement of Net Income as of December 31, 2022 - Unaudited
| (in K€) | IAS 19 | IFRS 16 | Pro forma adjustments |
|||||||||
| Revenues |
— | — | — | |||||||||
| Other income |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating income |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Research and development |
22 | (180 | ) | (157 | ) | |||||||
| Selling, general and administrative |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses |
22 | (180 | ) | (157 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Other operating income and expenses |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating income (loss) |
22 | (180 | ) | (157 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Financial income |
— | — | — | |||||||||
| Financial expenses |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Financial result |
— | — | — | |||||||||
| Income tax |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) |
22 | (180 | ) | (157 | ) | |||||||
|
|
|
|
|
|
|
|||||||
66
The total pro forma adjustments to the Consolidated Statement of Net Income amounted to €(157)K and consisted mainly of :
| • | €22K for the homogeneity adjustment of the pension commitment recalculated on the basis of Erytech’s calculation assumptions; |
| • | € (180)K for the adjustment of the duration of commitments on the Nantes and Romainville premises, consisting of a decrease in rights of use of €846K and a decrease in lease liabilities of €826K linked to the shortening of rental periods and €148K of additional depreciation on tangible fixed assets linked to the shortening of the lifespan of the fittings in Nantes. |
Note 4: Details of pro forma PHERECYDES adjustments to the Pro Forma Consolidated Statement of Financial Position as of December 31, 2022 - Unaudited - ASSETS
| (in K€) | IAS 19 | IFRS 16 | Pro forma adjustments |
|||||||||
| ASSETS |
||||||||||||
| Non-current assets |
||||||||||||
| Intangible assets |
— | — | — | |||||||||
| Goodwill |
— | — | — | |||||||||
| Property, plant and equipment |
— | (148 | ) | (148 | ) | |||||||
| Right of use |
— | (846 | ) | (846 | ) | |||||||
| Other non-current assets |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total non-current assets |
— | (995 | ) | (995 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Current assets |
||||||||||||
| Inventories |
— | — | — | |||||||||
| Trade and other receivables |
— | — | — | |||||||||
| Other current assets |
— | — | — | |||||||||
| Cash and cash equivalents |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL ASSETS |
— | (995 | ) | (995 | ) | |||||||
|
|
|
|
|
|
|
|||||||
67
Note 4: Details of pro forma PHERECYDES adjustments to the Pro forma Consolidated Statement of Financial Position as of December 31, 2022 - Unaudited - LIABILITIES AND SHAREHOLDERS’ EQUITY
| (in K€) | IAS 19 | IFRS 16 | Pro forma adjustments |
|||||||||
| LIABILITIES AND EQUITY |
||||||||||||
| Shareholders’ equity |
||||||||||||
| Share capital |
— | — | — | |||||||||
| Premiums related to share capital |
— | — | — | |||||||||
| Reserves |
11 | 11 | 22 | |||||||||
| Translation reserves |
— | — | — | |||||||||
| Net income (loss) |
22 | (180 | ) | (157 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total equity |
33 | (169 | ) | (136 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Non-current liabilities |
||||||||||||
| Provisions - non-current portion |
(33 | ) | — | (33 | ) | |||||||
| Financial liabilities - non-current portion |
— | — | — | |||||||||
| Derivative liabilities - non-current portion |
— | — | — | |||||||||
| Lease liabilities - non-current portion |
— | (856 | ) | (856 | ) | |||||||
| Deferred tax |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total non-current liabilities |
(33 | ) | (856 | ) | (889 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Current liabilities |
||||||||||||
| Provisions - current portion |
— | — | — | |||||||||
| Financial liabilities - current portion |
— | — | — | |||||||||
| Derivative liabilities - current portion |
— | — | — | |||||||||
| Lease liabilities - current portion |
— | 30 | 30 | |||||||||
| Trade and other payables |
— | — | — | |||||||||
| Current income tax liabilities |
— | — | — | |||||||||
| Other current liabilities |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
— | 30 | 30 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL LIABILITIES AND EQUITY |
(0 | ) | (995 | ) | (995 | ) | ||||||
|
|
|
|
|
|
|
|||||||
Adjustment of provisions for retirement benefits (IAS19)
The calculation of provisions for retirement benefits for PHERECYDES employees has been harmonized with the rules applied by Erytech (collective agreement, etc.).
Adjustment of the lease liability and the right of use (IFRS16)
Pro forma IFRS adjustments related to the lease contracts for PHERECYDES’ Nantes and Romainville premises have been recalculated using early surrender assumptions to take into account the planned combination of PHERECYDES’ Lyon teams with ERYTECH’s teams by December 31, 2023. For the Nantes premises, the 6-month notice period will be respected for December 31, 2023. On the other hand, the fixtures and fittings have been depreciated at an accelerated rate to arrive at a net value of
68
zero as of December 31, 2023. For the Romainville premises, the contract can only be terminated by September 30, 2024, which is why the debt is maintained until that date and the right of use is amortized until December 31, 2023. The discount rates used are the market rates closest to those of PHERECYDES at the date of the combination. The amortization period of the rights of use has been reduced to December 31, 2023 for both sites. The lease liabilities have been adjusted by € (826K). The right of use has been reduced by €1,024K to take into account the planned release of the premises on December 31, 2023.
The adjustments also include an acceleration of the depreciation charge for the Nantes premises in the amount of €148,000 as of December 31, 2022, due to the announced move.
Note 5: Tax impacts of IFRS and pro forma adjustments
ERYTECH and PHERECYDES are in structurally loss-making tax situations, with no prospect of taxable profits in the coming years, which does not allow for the recognition of deferred tax assets. In addition, no current tax effect has been recognized on the pro forma adjustments and transaction costs.
Note 6: PHERECYDES capital increase
This column presents the effects of the capital increase subscribed by the historical shareholders in February 2023. The main features of this capital increase are presented in the paragraph “Main terms of the merger project” (page 2).
Note 7: Business combination
When the business combination is not cash-settled, the consideration transferred is measured at the fair value of the securities issued as consideration for the contributions at the transaction date of the business combination. The fair value of the consideration transferred is likely to change until the completion date of the transactions since the securities issued as consideration for the contributions will be valued on the basis of the Erytech share price on the transaction date.
Details of the column “Business combination” in the pro forma consolidated statement of financial position as of December 31, 2022 - Unaudited
| (in K€) | Purchase of PHERECYDES shares (Contribution in kind) |
Purchase of additional PHERECYDES shares (Merger) |
IFRS 3 | Business combination |
||||||||||||
| ASSETS |
||||||||||||||||
| Non-current assets |
||||||||||||||||
| Goodwill |
29,197 | 29,197 | ||||||||||||||
| Equity securities |
2,956 | 25,327 | (28,283 | ) | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total non-current assets |
2,956 | 25,327 | 914 | 29,197 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Current assets |
||||||||||||||||
| Total current assets |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL ASSETS |
2,956 | 25,327 | 914 | 29,197 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
69
| (in K€) | Purchase of PHERECYDES shares (Contribution in kind) |
Purchase complementary titles PHERECYDES (Merger) |
IFRS 3 | Change in scope |
||||||||||||
| LIABILITIES AND EQUITY |
||||||||||||||||
| Shareholders’ equity |
||||||||||||||||
| Share capital |
310 | 2,658 | (7,939 | ) | (4,971 | ) | ||||||||||
| Premiums related to share capital |
2,646 | 22,669 | (9,686 | ) | 15,629 | |||||||||||
| Reserves |
9,649 | 9,649 | ||||||||||||||
| Net income |
8,890 | 8,890 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total shareholders’ equity |
2,956 | 25,327 | 914 | 29,197 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Non-current liabilities |
||||||||||||||||
| Total non-current liabilities |
— | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Current liabilities |
||||||||||||||||
| Trade and other payables |
— | |||||||||||||||
| Total current liabilities |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL LIABILITIES AND EQUITY |
2,956 | 25,327 | 914 | 29,197 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
SHARE PRICE USED TO CALCULATE THE TRANSACTION PRICE
The calculation of the transaction price used for the purposes of preparing the Pro Forma Financial Information was based on the Erytech share price on May 15, 2023 at €0.953. This date is as close as possible to the date of preparation of this document. The final price to be used will only be known after the merger by the end of the second quarter of 2023.
The calculation of the parity of 15 ERYTECH shares for 4 PHERECYDES shares was carried out on the basis of the stock market price on January 19, 2023 (date of the agreement between the two companies), this calculation valued each company at an equivalent amount of €18.9M.
The price of the transaction is extremely sensitive to the evolution of ERYTECH’s share price, which changes often as shown by the examples of share prices shown below.
| Dates or Period | Price ERYTECH (€) |
Price PHERECYDES (€) |
Ratio Parity |
Number of titles ERYTECH to be created |
Transaction price |
Goodwill | Variation Goodwill |
Variation in % |
||||||||||||||||||||||||||||
| Historical |
19/01/2023 | 0.61 | 2.29 | 3.75 | 29,677,639 | 18,103 | 19,017 | (10,179 | ) | -35 | % | |||||||||||||||||||||||||
| Pro forma |
15/05/2023 | 0.95 | 2.12 | 3.75 | 29,677,639 | 28,283 | 29,197 | — | — | |||||||||||||||||||||||||||
| Minimum |
Last 12 months | 0.34 | 1.91 | 3.75 | 29,677,639 | 10,031 | 10,945 | (18,252 | ) | -63 | % | |||||||||||||||||||||||||
| Average |
Last 12 months | 0.87 | 2.91 | 3.75 | 29,677,639 | 25,868 | 26,782 | (2,415 | ) | -8 | % | |||||||||||||||||||||||||
| Maximum |
Last 12 months | 1.39 | 4.98 | 3.75 | 29,677,639 | 41,252 | 42,166 | 12,969 | 44 | % | ||||||||||||||||||||||||||
70
Provisional allocation of the purchase price: The entry into the scope of consolidation related to the merger does not include a fair value measurement of the assets, liabilities and contingent liabilities of PHERECYDES, with the exception of the items mentioned in note 4, which have been presented as pro forma adjustments. The final assessment of the fair value of the assets, liabilities and contingent liabilities of PHERECYDES will be conducted after the completion of the Transactions. Consequently, the determination of goodwill, based on the financial statements of the PHERECYDES Group as of December 31, 2022, is provisional and is performed solely for the purpose of preparing the Pro Forma Financial Information. It may therefore be subject to subsequent changes depending on the valuations that will be carried out for the purposes of preparing the consolidated financial statements for the fiscal year 2023.
ESTIMATED CONSIDERATION TRANSFERRED
Under IFRS, the fair value at the acquisition date of the consideration transferred by the accounting acquirer is determined on the basis of the number of shares that Erytech, the accounting acquirer, would have had to issue to PHERECYDES’ shareholders.
For the purposes of the Unaudited Pro Forma Condensed Consolidated Financial Information, the consideration transferred has been determined on the basis of a conversion of all the shares of PHERECYDES.
Based on the above information, the preliminary consideration transferred is determined as follows:
| Number of ERYTECH shares outstanding as of December 31, 2022 |
31,018,553 | |||||
|
|
|
|||||
| Total number of Erytech shares outstanding as of December 31, 2022 |
31 018 553 | A | ||||
| Number of shares comprising the capital of PHERECYDES as of December 31, 2022 |
7,221,477 | |||||
| Capital increase on February 15, 2023 |
717,702 | |||||
| Treasury shares |
(25,142 | ) | ||||
|
|
|
|||||
| Total number of PHERECYDES shares to be considered for the merger |
7,914,037 | B | ||||
| Exchange ratio (including dilutive instruments) |
3.75 | C | ||||
| Number of shares after reconciliation |
60,696,192 | A+(B*C) | ||||
| Contribution of 827,132 PHERECYDES shares |
827,132 | |||||
| ERYTECH share equivalent |
3,101,745 | D | ||||
| Contribution of the remaining PHERECYDES shares at the time of the merger |
7,086,905 | |||||
| ERYTECH share equivalent |
26,575,894 | E | ||||
|
|
|
|||||
| Total ERYTECH capital increase |
29 677 639 | D+E |
The number of ERYTECH shares to be created as consideration for the contribution of the PHERECYDES shares is 29,677,639.
The valuation used is the stock market price on May 15, 2023, i.e. €0.953, which gives a valuation of the contribution of €28,283K.
71
Preliminary goodwill has been calculated on the basis of the estimated value of the consideration transferred and the assets acquired and liabilities assumed:
| Fair value of identifiable assets and liabilities at the transaction date |
31/12/2022 | Capital increase 02/17/2023 | Total | |||||||||
| Intangible assets |
31 | — | 31 | |||||||||
| Property, plant and equipment |
411 | — | 411 | |||||||||
| Rights of use |
592 | — | 592 | |||||||||
| Other non-current assets |
125 | — | 125 | |||||||||
| Other current assets |
2,399 | — | 2,399 | |||||||||
| Cash and cash equivalents |
1,035 | 1,500 | 2,535 | |||||||||
| Financial debts |
-3,012 | — | -3,012 | |||||||||
| Lease liabilities |
-650 | — | -650 | |||||||||
| Other non-current liabilities |
-41 | — | -41 | |||||||||
| Other current liabilities |
-3,303 | — | -3,303 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net assets acquired |
-2,414 | 1,500 | -914 | |||||||||
| Purchase price |
28,283 | |||||||||||
|
|
|
|||||||||||
| Goodwill |
29,197 | |||||||||||
|
|
|
|||||||||||
Once the valuation work in the context of the accounting for the business combination within the meaning of IFRS 3 is completed, these values will be adjusted.
The summary of PHERECYDES’ equity at the transaction date is as follows:
| (in K€) | PHERECYDES in ERYTECH presentation |
IFRS transition adjustments PHERECYDES |
Pro forma adjustments |
Capital increase PHERECYDES |
Transaction costs |
Total shareholders’ equity at transaction date |
||||||||||||||||||
| LIABILITIES AND EQUITY |
||||||||||||||||||||||||
| Shareholders’ equity |
||||||||||||||||||||||||
| Share capital |
7,221 | — | 718 | 7,939 | ||||||||||||||||||||
| Premiums related to share capital |
8,904 | — | 782 | 9,686 | ||||||||||||||||||||
| Reserves |
(3,749 | ) | (5,923 | ) | 22 | (9,649 | ) | |||||||||||||||||
| Translation reserves |
— | — | — | |||||||||||||||||||||
| Net income (loss) |
(4,854 | ) | (3,198 | ) | (157 | ) | (680 | ) | (8,890 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total equity |
7,522 | (9,121 | ) | (136 | ) | 1,500 | (680 | ) | (914 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Note 8: Transaction costs
No transaction costs were incurred by ERYTECH or PHERECYDES during the year ended December 31, 2022.
Transaction costs borne by ERYTECH
The costs related to the Transactions in 2023 have been estimated and included in the pro forma financial information. The costs related to the merger borne by Erytech have been expensed in the income statement for an amount of €3,073,000, under selling, general and administrative.
72
Transaction costs borne by PHERECYDES
The costs related to the Transactions in 2023 estimated by PHERECYDES are €680K and are reflected in the pro forma accounts in the net assets of the latter.
| 5.7 | REPORT OF THE STATUTORY AUDITORS OF ERYTECH ON THE PRO FORMA FINANCIAL INFORMATION |
|
KPMG S.A. 51 rue de Saint-Cyr CS 60409 69338 Lyon Cedex 9 France |
RSM Paris 26 rue Cambacérès 75008 Paris |
ERYTECH PHARMA S.A.
60 avenue Rockefeller
69008 LYON
Statutory auditors’ report on the Pro Forma Financial Information
for the year ended December 31, 2022
This is a free translation into English of the statutory auditors’ report issued in the French language and is provided solely for the convenience of English speaking readers.
This report should be read in conjunction with, and is construed in accordance with, French law and professional standards applicable in France.
Erytech Pharma S.A.
Registered office: 60 avenue Rockefeller, 69008 LYON
Statutory auditors’ report on the Pro Forma Financial Information for the year ended December 31, 2022
To the Chief Executive Officer,
In our capacity as statutory auditors of your company and in accordance with Regulation (EU) n°2017/1129 supplemented by the Commission Delegated Regulation (EU) n°2019/980 and the Commission Delegated Regulation (EU) n° 2021/528, we hereby report to you on the pro forma financial information of Erytech Pharma S.A. (the “Company”) for the year ended December 31, 2022 set out in section 5.6 of the exemption document prepared for the merger-absorption of Pherecydes Pharma S.A. by the Company (the “Pro Forma Financial Information”).
73
The Pro Forma Financial Information has been prepared for the sole purpose of illustrating the impact that “the Operations” (all the operations which are expected in the the above mentioned context, i.e. the merger-absorption of Pherecydes Pharma S.A. by the Company and the share capital increase of Pherecydes Pharma S.A.prior to the merger, it being understood that these operations cannot be seperated) might have had on the consolidated balance sheet at December 31, 2022 and the consolidated income statement of the Company for the year ended December 31, 2022 had it taken place with effect as of December 31, 2022 for the consolidated balance sheet and from Januray 1, 2022 for the consolidated income statement. By its very nature, this information is based on a hypothetical situation and does not represent the financial position or performance that would have been reported, had the Operations or events taken place at an earlier date than the actual or contemplated date.
It is your responsibility to prepare the Pro Forma Financial Information in accordance with the provisions of Regulation (EU) n°2017/1129 and the Commission Delegated Regulation (EU) 2021/528 and ESMA’s recommendations on Pro Forma Financial Information.
It is our responsibility to express a conclusion, based on our work, in accordance with Annex I, section 5.9 of the Commission Delegated Regulation (EU) n°2021/528, as to the proper compilation of the Pro Forma Financial Information on the basis stated.
We performed those procedures that we deemed necessary according to the professional guidance of the French Institute of Statutory Auditors (“CNCC”) applicable to such engagement. These procedures, which did not include an audit or a review of the financial information used as a basis to prepare the Pro Forma Financial Information, mainly consisted in ensuring that the information used to prepare the Pro Forma Financial Information was consistent with the underlying financial information, as described in the notes to the Pro Forma Financial Information, reviewing the evidence supporting the pro forma adjustments and conducting interviews with the management of the Company to obtain the information and explanations that we deemed necessary.
In our opinion:
| a) | the Pro Forma Financial Information has been properly compiled on the basis stated; |
| b) | that basis is consistent with the accounting policies of the Company. |
This report has been issued solely for the purposes of the filing of the exemption document with the French financial markets authority (Autorité des marchés financiers or “AMF”) and cannot be used for any other purpose.
The statutory auditors
French original signed by KPMG S.A. and RSM Paris on May 23, 2023.
74
| 6. | AVAILABLE DOCUMENTS |
The K-bis and articles of association of the Absorbing Company and the Absorbed Company are available free of charge at the registered offices of the companies (ERYTECH Pharma, 60, avenue Rockefeller, 69008 Lyon and Pherecydes Pharma, 22, boulevard Benoni Goullin, 44200 Nantes).
In addition, it should be noted that the Exemption Document incorporates by reference:
| • | with respect to Erytech: the Erytech 2022 Universal Registration Document, available on Erytech’s website (https://erytech.com/fr/investisseurs/amf-information-reglementee/document-de-reference/). |
| • | with respect to Pherecydes: the Pherecydes 2022 Annual Financial Report (including the Pherecydes Corporate Governance Report), available on Pherecydes’ website (https://www.pherecydes-finance.com/fr/documentation/presentations). |
75
ANNEX 1. MERGER AGREEMENT
76
TRANSLATION FOR INFORMATION PURPOSE ONLY
DRAFT MERGER AGREEMENT
between
ERYTECH PHARMA
and
PHERECYDES PHARMA
Dated May 15, 2023
CONTENTS
| 1. | DEFINITIONS - INTERPRETATION |
80 | ||||
| 2. | COMPANY PRESENTATION |
81 | ||||
| 3. | REASONS AND PURPOSES OF THE MERGER |
83 | ||||
| 4. | CONSULTATION OF THE INSTITUTIONS REPRESENTING THE PERSONNEL |
83 | ||||
| 5. | EFFECTIVE DATE - COMPLETION DATE - OWNERSHIP AND USE |
84 | ||||
| 6. | ACCOUNTS OF THE INTERESTED COMPANIES USED TO ESTABLISH THE TERMS OF THE MERGER |
84 | ||||
| 7. | MERGER REGIME |
85 | ||||
| 8. | VALUATION OF THE CONTRIBUTION |
85 | ||||
| 9. | MERGER AUDITOR |
85 | ||||
| 10. | CHANGES MADE OR TO BE MADE BEFORE THE DATE OF COMPLETION |
86 | ||||
| 11. | MERGER OF THE ABSORBED COMPANY INTO THE ABSORBING COMPANY |
87 | ||||
| 12. | UNDERTAKING OF THE PARTIES |
92 | ||||
| 13. | REPRESENTATIONS OF THE PARTIES |
92 | ||||
| 14. | SHARES EXCHANGE RATIO AND REMUNERATION OF THE CONTRIBUTION |
93 | ||||
| 15. | DISSOLUTION OF THE ABSORBED COMPANY |
97 | ||||
| 16. | CONDITIONS PRECEDENT TO THE COMPLETION OF THE MERGER AND SUBSEQUENT TRANSACTIONS |
97 | ||||
| 17. | TAX RETURNS AND OBLIGATIONS |
97 | ||||
| 18. | MISCELLANEOUS |
101 | ||||
| LIST OF SCHEDULES |
105 | |||||
78
BETWEEN THE UNDERSIGNED:
| (1) | ERYTECH PHARMA, a French société anonyme whose registered office is located at 60, avenue Rockefeller, 69008 Lyon, France, registered in the Lyon Trade and Companies Register under number 479 560 013, represented by its Managing Director, Mr. Gil Beyen, duly authorized for the purposes of signing the present document, |
hereinafter referred to as “Erytech” or the “Absorbing Company”
| (2) | PHERECYDES PHARMA, a French société anonyme whose registered office is located at 22 boulevard Benoni Goullin, 44200 Nantes, France, registered in the Nantes Trade and Companies Register under number 493 252 266, represented by its Managing Director, Mr. Thibaut du Fayet, duly authorized for the purpose of signing the present document, |
hereinafter referred to as “Pherecydes” or the “Absorbed Company”
The Absorbing Company and the Absorbed Company are hereinafter individually referred to as a “Party” and collectively as the “Parties”.
IT WAS PREVIOUSLY STATED THAT:
| (A) | Erytech is a clinical-stage biotechnology company, founded in 2004, that develops innovative treatments, derived from in-house research and development programs, to cure patients suffering from diseases in therapeutic areas with unmet needs, based in particular on red blood cells. The ordinary shares of Erytech (the “Erytech Shares”) are listed on the regulated market of Euronext Paris (“Euronext Paris”) under ISIN code FR0011471135 and, in the form of American Depositary Shares, on the Nasdaq Securities Market LLC (“Nasdaq”). |
| (B) | Pherecydes is a clinical-stage biotechnology company, created in 2006, which develops innovative treatments, resulting from the internal conduct of research and development programs, to cure patients suffering from diseases in therapeutic areas with unmet needs and based notably on bacteriophages. The ordinary shares of Pherecydes (the “Pherecydes Shares”) are listed on the Euronext Growth multilateral trading facility (“Euronext Growth”) under ISIN code FR0011651694. |
| (C) | Pursuant to the memorandum of understanding dated February 15, 2023 (the “MoU”) relating to the proposed merger of Pherecydes into Erytech (the “Merger”), the Parties have agreed to enter into the present draft merger agreement (the “Merger Agreement”), the purpose of which is to determine the terms and conditions of the Merger. |
| (D) | On March 20, 2023, the Social and Economic Committee (the “SEC”) of Erytech gave its opinion on the Merger. |
79
WITH THIS IN MIND, IT WAS AGREED AS FOLLOWS:
| 1. | DEFINITIONS - INTERPRETATION |
| 1.1 | Definitions |
For purposes of the Merger Agreement, the following terms beginning with a capital letter shall have the meanings set forth below:
“Absorbed Company” has the meaning set forth in the recitals;
“Absorbing Company” has the meaning set forth in the recitals;
“AMF Decision” has the meaning set forth in Article 16;
“BSPCE” the meaning set forth in Article 14.5;
“Completion Date” has the meaning set forth in Article 16;
“Contributions in Kind” shall have the meaning set forth in Article 10.1;
“Effective Date” has the meaning set forth in Article 5.2;
“Erytech” has the meaning set forth in the recitals;
“Erytech Shares” has the meaning set forth in paragraph (A) of the Preamble;
“Euronext Growth” shall have the meaning set forth in paragraph (B) of the Preamble;
“Euronext Paris” has the meaning set forth in paragraph (A) of the Preamble;
“Merger” has the meaning set forth in paragraph (C) of the Preamble;
“Merger Agreement” has the meaning set forth in paragraph (C) of the Preamble;
“Merger Auditor” has the meaning set forth in Article 9;
“MFA” has the meaning set forth in Article 16;
“MoU” has the meaning set forth in paragraph (C) of the Preamble;
“Nasdaq” has the meaning set forth in paragraph (A) of the Preamble;
“NCA” has the meaning set forth in Article 8;
“New Shares” has the meaning set forth in Article 14.2;
“Parties” has the meaning set forth in the recitals;
“Pherecydes” has the meaning set forth in the recitals;
80
“Pherecydes Shares” has the meaning set forth in paragraph (B) of the Preamble;
“Preamble” means the preamble to the Merger Agreement;
“Reference Accounts of the Absorbed Company” has the meaning set forth in Article 6;
“Reference Accounts of the Absorbing Company” has the meaning set forth in Article 6;
“SEC” has the meaning set forth in paragraph (D) of the Preamble;
“Treasury Shares” means the 25,142 Pherecydes Shares held by the Absorbed Company as of the date hereof.
| 1.2 | Interpretation |
In the Merger Agreement, unless the context otherwise requires and unless otherwise expressly provided:
| (a) | Any reference to Articles and Schedules is to the articles or schedules of the Merger Agreement; |
| (b) | the meaning attributed to the terms defined in the Merger Agreement shall apply to both the singular and plural of such terms and, where applicable, to their other grammatical forms; |
| (c) | the headings of the Articles and Schedules of the Merger Agreement have been inserted only for ease of reading and do not affect their meaning or interpretation; |
| (d) | The word “or” has a disjunctive meaning, not an alternative meaning (i.e., when two elements or qualities are separated by the word “or”, the existence of one of these elements or qualities is not supposed to exclude the existence of the other and the word “or” is supposed to include the word “and”); |
| (e) | the terms “including” or “in particular” and any other terms having the same meaning are not limiting; and |
| (f) | unless otherwise specified, any reference to a contract, commitment, agreement or understanding is to any contract, commitment, agreement or understanding creating rights or obligations, whether in written or oral form. |
| 2. | COMPANY PRESENTATION |
| 2.1 | The Absorbed Company |
| 2.1.1 | Pherecydes is a French société anonyme registered on 23 March 2010 for a period of 99 years unless extended or dissolved early. |
The purpose of the Absorbed Company is, in France and abroad, to develop know-how, obtain patents and licenses in the field of biology, medicine and more generally in the field of life sciences, on its own behalf or on behalf of third parties, with a view to marketing and distributing its products, as well as any service activity related to the field of life sciences.
81
And generally, all financial, commercial, industrial, movable and immovable operations, which may be directly or indirectly related to the above object or to any similar or related objects, likely to favour its extension or development, including any agreement of any kind with industrialists or investors.
Its share capital amounts to EUR 7,939,179. It is divided into 7,939,179 Pherecydes shares of one (1) euro per value each, fully paid up and all of the same class.
Its financial year begins on 1st January and ends on 31st December of the following year.
The Absorbed Company is subject to corporate income tax in France.
| 2.1.2 | The shareholders of the Absorbed Company do not have any special rights or benefits. |
Except for the dilutive instruments listed in the Schedule 2.1.2, the Absorbed Company has not issued any securities giving access, by conversion, exchange, redemption, exercise of a security, or in any other way, immediately or in the future, to its capital.
In order to facilitate the Merger, the board of directors of the Absorbed Company has decided, as from the date hereof and until the Completion Date, not to use the share repurchase program and not to cancel the Treasury Shares.
| 2.2 | The Absorbing Company |
| 2.2.1 | Erytech is a French société anonyme registered on 22 November 2004 for a period of 99 years unless extended or dissolved early. |
The purpose of the Absorbing Company is in France and in all countries:
| • | Research, manufacture, import, distribution and marketing of investigational drugs, medicines, medical devices and apparatus. |
| • | The realization of all consulting services related to it. |
And generally, any financial, commercial, industrial, civil, real estate or movable operations, which may be directly or indirectly related to one of the specified objects or likely to facilitate the realization thereof.
The Absorbing Company may act directly or indirectly and carry out all these operations in all countries, on its own behalf or on behalf of third parties, either alone or with third parties in participation, association, grouping or company, by way of creation of new companies, contribution, limited partnership, subscription, purchase of securities or corporate rights, merger, alliance, joint venture or taking or giving in lease or in management of all goods and rights or otherwise
82
Its capital is currently EUR 3,101,855.30. It is divided into 31,018,553 Erytech Shares of ten cents of euro (EUR 0.10) each, fully paid up and all of the same class.
Its financial year begins on 1st January and ends on 31st December of the following year.
The Absorbing Company is subject to corporate income tax in France.
| 2.2.2 | The shareholders of the Absorbing Company do not have any special rights or advantages, except for a double voting right on the Erytech Shares held for more than two years in registered form. |
Except for the dilutive instruments listed in the Schedule 2.2.2, the Absorbing Company has not issued any securities giving access, by conversion, exchange, redemption, exercise of a security, or in any other way, immediately or in the future, to its capital.
| 2.3 | Relationship of companies to each other |
| 2.3.1 | Capital links |
As of the date of this Merger Agreement, to the best of their knowledge, the Absorbing Company and the Absorbed Company do not have any capital link between them, except for the links with respect to the Contributions in Kind to be made, as set forth in Article 10.1.
| 2.3.2 | Common manager |
As of the date of this Merger Agreement, the Absorbing Company and the Absorbed Company do not have any common manager.
| 3. | REASONS AND PURPOSES OF THE MERGER |
The Merger is part of a strategic combination aimed at creating a world leader in phage therapy. The Merger aims to capitalize on the financial resources and teams of the Absorbed Company and the Absorbing Company to both accelerate and expand Pherecydes’ existing phage therapy development programs, launch new phage candidates and potentially broaden the scope of application to new therapeutic modalities by leveraging the advanced technology platforms and expertise of both companies.
As part of the Merger, it is planned to merge the activities of the Absorbing Company and the Absorbed Company and to relocate all the teams to Erytech’s premises in Lyon, France, where they will benefit from a location within a major European cluster in the field of infectious diseases.
| 4. | CONSULTATION OF THE INSTITUTIONS REPRESENTING THE PERSONNEL |
In accordance with Article L. 2312-8 of the French Labor Code, the Erytech SEC has, prior to the signature of the Merger Agreement, been informed and consulted on the proposed Merger.
The notice of the transaction was given on March 20, 2023.
83
| 5. | EFFECTIVE DATE - COMPLETION DATE - OWNERSHIP AND USE |
| 5.1 | Date of completion Legal status of the Merger |
The Merger will be definitively completed on the Completion Date, as such term is defined in Article 16, subject to and solely as a result of the lifting of the conditions precedent referred to in such Article.
The Absorbing Company shall own the assets and liabilities contributed by the Absorbed Company, including those that would have been omitted either in the draft Merger Agreement or in the accounts of the Absorbed Company, as from the Completion Date, and the assets and liabilities of the Absorbed Company shall be vested in the Absorbing Company in the same state as they will be on such date.
| 5.2 | Use of the merger-contribution - Effective Date |
Without prejudice to the provisions of article 3.8 of the MoU, until the Completion Date, Pherecydes undertakes to manage the contributed assets and rights with the same principles, rules and conditions as in the past, not to make any material commitment or commitment that may affect the ownership or free disposal of such assets, all without the prior consent of Erytech (which consent shall not be unreasonably withheld).
The Absorbing Company shall be deemed to have the benefit of the assets and liabilities contributed by the Absorbed Company retroactively as of the Effective Date, as well as, more generally, any asset or liability not known or foreseeable as of that date, and all transactions to which the transferred items may have been subject during the interim period shall be automatically deemed to have been carried out for the account and at the risk of the Absorbing Company.
From an accounting and tax point of view, the Merger will take effect retroactively as of January 1st, 2023 (the “Effective Date”); consequently, the results of all active and passive transactions, carried out by the Absorbed Company as of January 1st, 2023 until the Completion Date, will be exclusively for the benefit of or at the expense of the Absorbing Company and will be deemed to be carried out by the Absorbing Company.
| 6. | ACCOUNTS OF THE INTERESTED COMPANIES USED TO ESTABLISH THE TERMS OF THE MERGER |
The terms and conditions of the Merger are established on the basis of:
| • | for the Absorbing Company, the annual financial statements for the financial year ending December 31, 2022 approved by the board of directors of the Absorbing Company on March 22, 2023 and certified by the statutory auditors on March 28, 2023, as they appear in the 2022 universal registration document of the Absorbing Company, filed with the French financial markets authority (AMF) on March 29, 2023 under number D. 23-0172, which can be consulted on the website of the Absorbing Company5 (the “Reference Accounts of the Absorbing Company”); and |
| 5 | To the following address: https://investors.erytech.com/static-files/f6543ff2-4348-42c8-9480-b52145272098. |
84
| • | for the Absorbed Company, the annual financial statements for the financial year ended December 31, 2022, approved by the board of directors of the Absorbed Company on March 30, 2023 and certified by the statutory auditors on April 26, 2023, as set out in the Schedule 6 (the “Reference Accounts of the Absorbed Company”). |
| 7. | MERGER REGIME |
As this is a merger between companies in the form of a French société anonyme, the Merger will be carried out pursuant to the provisions of Articles L. 236-1 to L. 236-6 and L. 236-8 toL. 236-15 of the French Commercial Code.
| 8. | VALUATION OF THE CONTRIBUTION |
As this is an “up-side-down” transaction involving entities under separate control, within the meaning of Autorité des normes comptables (the “ANC”) Regulation No. 2014-03 relating to the general chart of accounts of 5 June 2014, as last amended by ANC Regulation No. 2014-03 relating to the general chart of accounts of June 5, 2014, as last amended by ANC regulationn° 2022-01 of March 11, 2022, the Parties have agreed to retain as the contribution value of the assets contributed by the Absorbed Company and of the liabilities assumed by the Absorbing Company in the context of the Merger, their real value.
The valuation methods used to determine the fair value of assets and liabilities are detailed in the Schedule 14.1.
On this basis, the real value of the assets contributed by the Absorbed Company to the Absorbing Company amounts to EUR 16,537,386, as shown in the designations and valuations of the assets and liabilities contributed below.
| 9. | MERGER AUDITOR |
The President of the Commercial Court of Lyon has, by order dated February 28, 2023, appointed as merger auditor the firm Finexsi, a French société anonyme whose registered office is located at 14 rue de Bassano 75016 Paris, registered in the Paris Trade and Companies Register under the number 412 029 357, in the person of Mr. Christophe Lambert (the “Merger Auditor”)
The Merger Auditor is responsible:
| • |
|
| • | to examine the terms and conditions of the Merger; |
85
| • | to assess the value of the contributions in kind and, if applicable, of the special benefits that may be granted and to verify that the relative values attributed to the Absorbing Company and the Absorbed Company are relevant and that the exchange ratio is fair; and |
| • | to prepare the reports, containing the information required by the applicable regulations, which will be made available to the shareholders of the Absorbing Company and the Absorbed Company under the conditions defined by the legal and regulatory provisions in force. |
| 10. | CHANGES MADE OR TO BE MADE BEFORE THE DATE OF COMPLETION |
| 10.1 | Significant transactions affecting the capital of the Parties between the date of signature of this Merger Agreement and the Completion Date |
Prior to the date of signature of the Merger Agreement, the following shareholders of the Absorbed Company:
| • | AURIGA IV BIOSEEDS, a French professional private equity fund represented by ELAIA PARTNERS (443 990 668 RCS Paris); |
| • | OUEST VENTURES III, a French professional private equity fund represented by GO CAPITAL (445 284 458 RCS Rennes); |
| • | Mr. Guy Rigaud and certain investors listed in the Schedule 10.1; |
representing together approximately 46.81% of the share capital and voting rights of the Absorbed Company,
and the Absorbing Company entered into a contribution in kind agreement pursuant to which they agreed to contribute, prior to the Completion Date, 827,132 ordinary shares of the Absorbed Company, in accordance with the support commitments entered into in connection with the execution of the MoU, pursuant to which such shareholders of the Absorbed Company agreed to (i) contribute, prior to the Completion Date, 827,132 Pherecydes Shares to the Absorbing Company in consideration for newly issued Erytech Shares, at the same exchange ratio as the Merger (the “Contributions in Kind”) and to (ii) participate in the general shareholders’ meeting of the Absorbing Company convened to approve the proposed Merger and vote in favor of the proposed Merger at such meeting.
An increase of the share capital of the Absorbing Company by the issuance of 3,101,745 new Erytech Shares as consideration for the Contributions in Kind will be decided, prior to the Completion Date, by the board of directors of the Absorbing Company in accordance with the delegation of authority granted by the general shareholders’ meeting of the Absorbing Company on June 24, 2022 (29th resolution).
| 10.2 | Modification of the corporate name of the Absorbing Company |
The corporate name of the Absorbing Company will be changed on the Completion Date.
86
| 11. | MERGER OF THE ABSORBED COMPANY INTO THE ABSORBING COMPANY |
| 11.1 | Scope of the contribution of all the assets and liabilities of the Absorbed Company |
The Absorbed Company contributes, by way of merger, to the Absorbing Company, which accepts it, and under the ordinary of both fact and law guarantees in such matters, all the assets and liabilities making up its assets, as designated below, it being specified that:
| • | the assets contributed to the Absorbing Company and the liabilities assumed by it are those included in the assets and liabilities of the Absorbed Company as of the Effective Date used for the determination of the conditions of the Merger as set forth in Article 5.2 and that they may have changed since then; |
| • | the list of assets and liabilities referred to below in Article 11.2 is only indicative and is in principle non-limitative and that, in accordance with the provisions of Article L. 236-3 of the French Commercial Code, by the sole fact of the completion of the Merger, and the resulting universal transfer of the assets and liabilities of the Absorbed Company, all the assets and liabilities of the Absorbed Company will be transferred to the Absorbing Company in the state in which they will be at the Completion Date. |
In addition, the Merger of the Absorbed Company is granted and accepted under terms and conditions and subject to the allocations stipulated hereinafter.
| 11.2 | Designation and valuation of the assets contributed by the Absorbed Company |
The Merger of the Absorbed Company includes all the assets of this company for their real value as determined hereinafter (the net book value being given for information purposes only):
| Assets |
Net book value (EUR) |
Real value (EUR) |
||||||
| Intangible assets |
9 071 772 | 18 087 000 | ||||||
| Commercial fund |
0 | 1 017 000 | ||||||
| Other intangible assets |
3 763 640 | 0 | ||||||
| Establishment costs |
0 | 0 | ||||||
| Development costs |
5 277 049 | 17 070 000 | ||||||
| Concessions, patents and similar rights |
31 083 | 0 | ||||||
| Advances on intangible assets |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Property, plant and equipment |
559 184 | 559 184 | ||||||
| Land |
0 | 0 | ||||||
| Buildings |
0 | 0 | ||||||
| Technical installations, equipment |
135 094 | 135 094 | ||||||
| Other tangible assets |
424 090 | 424 090 | ||||||
| Assets under construction |
0 | 0 | ||||||
| Advances and deposits |
0 | 0 | ||||||
87
| Assets |
Net book value (EUR) |
Real value (EUR) |
||||||
| Financial assets |
149 825 | 149 825 | ||||||
| Participation according to the meq method |
0 | 0 | ||||||
| Other participations |
0 | 0 | ||||||
| Receivables related to equity investments |
0 | 0 | ||||||
| Other fixed assets |
25 269 | 25 269 | ||||||
| Loans |
0 | 0 | ||||||
| Other financial assets |
124 556 | 124 556 | ||||||
|
|
|
|
|
|||||
| Total fixed assets |
9 780 782 | 18 796 009 | ||||||
|
|
|
|
|
|||||
| Stock |
0 | 0 | ||||||
| Raw materials, supplies |
0 | 0 | ||||||
| In the process of producing goods |
||||||||
| Intermediate and finished products |
0 | 0 | ||||||
| Goods |
0 | 0 | ||||||
| 0 | 0 | |||||||
|
|
|
|
|
|||||
| Receivables |
2 367 790 | 2 367 790 | ||||||
| Advances and deposits paid on orders |
0 | 0 | ||||||
| Trade receivables and related accounts |
126 604 | 126 604 | ||||||
| Other receivables |
2 241 186 | 2 241 186 | ||||||
| Unpaid subscribed and called capital |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Availability |
1 035 127 | 1 035 127 | ||||||
| Marketable securities |
0 | 0 | ||||||
| Availability |
1 035 127 | 1 035 127 | ||||||
| Prepaid expenses |
32 412 | 32 412 | ||||||
|
|
|
|
|
|||||
| Total current assets |
3 435 329 | 3 435 329 | ||||||
|
|
|
|
|
|||||
| Asset translation differences |
0 | 0 | ||||||
The real value of the assets included in the contribution therefore amounts to EUR 22,231,338.
88
| 11.3 | Designation and valuation of liabilities assumed by the Absorbing Company |
The Merger of the Absorbed Company is granted and accepted in consideration of the assumption by the Absorbing Company of all the liabilities of the Absorbed Company, namely:
| Liabilities |
Net book value (EUR) |
Real value (EUR) |
||||||
| Provisions for risks and charges |
0 | 0 | ||||||
| Provisions for risks |
0 | 0 | ||||||
| Provisions for expenses |
0 | 0 | ||||||
|
|
|
|
|
|||||
| Financial debts |
3 070 146 | 3070 146 | ||||||
| Convertible bonds |
0 | 0 | ||||||
| Other bonds |
360 000 | 360 000 | ||||||
| Loans and debts with credit institutions |
2 178 264 | 2178 264 | ||||||
| Miscellaneous loans and financial debts |
531 882 | 531 882 | ||||||
|
|
|
|
|
|||||
| Operating liabilities |
2 383 806 | 2 383 806 | ||||||
| Advances and deposits received on orders in progress |
0 | 0 | ||||||
| Trade payables and related accounts |
1 589 993 | 1589 993 | ||||||
| Tax and social security liabilities |
791 278 | 791 278 | ||||||
| Payables on fixed assets and related accounts |
0 | 0 | ||||||
| Other debts |
2 535 | 2 535 | ||||||
| Deferred income |
240 000 | 240 000 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
5 693 952 | 5 693 952 | ||||||
|
|
|
|
|
|||||
| Foreign currency translation liabilities |
0 | 0 | ||||||
The total amount of liabilities included in the contribution is therefore assumed in the amount of EUR 5,693,952.
| 11.4 | Determination of the net assets contributed by the Absorbed Company |
From the above designations and valuations, it follows that:
| Net assets |
Real value (EUR) | |||
| The assets are contributed by the Contributing Company for a value of: |
22 231 338 | |||
| The liabilities assumed by the Absorbing Company amount to: |
5 693 952 | |||
|
|
|
|||
| Total net assets |
16 537 386 | |||
|
|
|
|||
The net assets contributed by the Absorbed Company, on the basis of 100% of the Pherecydes Shares, amount to: EUR 16,537,386 (which represents a net asset value of EUR 16,479,810.82 for the contributed Pherecydes Shares, after deduction from the Treasury Shares).
As provided in Article 14.3.1, the net assets contributed by the Absorbed Company and remunerated by the Absorbing Company pursuant to article L. 236-3 II of the French Commercial Code (subject to the completion of the Contributions in Kind indicated in Article 10.1), amounts to: EUR 14,757,430.84.
89
| 11.5 | Off-balance sheet commitments |
Independently of the above-mentioned assets and liabilities, the Absorbing Company will benefit, if necessary, from the commitments received by the Absorbed Company described in particular in the Reference Accounts of the Absorbing Company and will be substituted for the Absorbed Company in the burden of the commitments given, if necessary, by the latter and described in particular in the Reference Accounts of the Absorbed Company in the Schedule 6.
| 11.6 | Opposition of the creditors |
As provided in Article 11.3, the contribution by way of Merger of the Absorbed Company to the Absorbing Company is made at the expense of the Absorbing Company to pay all the liabilities of this company in discharge of the Absorbed Company.
These liabilities will be borne by the Absorbing Company, which will be liable for these debts in place of the Absorbed Company, without this substitution entailing novation with regard to the creditors.
As a consequence, pursuant to the provisions of Articles L. 236-14 and R. 236-8 of the French Commercial Code, the creditors of the Absorbed Company and of the Absorbing Company whose claim will be prior to the publication of the present Merger Agreement will be able to object within a period of thirty (30) days as from the publication of the present Merger Agreement.
| 11.7 | Charges and general conditions of the Merger |
The Merger is granted and accepted under the ordinary and legal conditions, including those described hereinafter.
| (a) | The Absorbing Company shall take the contributed assets and rights in the state in which they will be on the Completion Date without being able to exercise any recourse whatsoever, for any reason whatsoever, against the Absorbed Company, in particular for wear and tear or poor condition of the equipment and movable objects, error in the designations or in their content (whatever the error may be), insolvency of the debtors or any other cause. |
| (b) | The Absorbing Company will be purely and simply substituted in all the rights and obligations of the Absorbed Company, which does not intend to give any other guarantee than those held by itself. |
| (c) | In accordance with the provisions of Article L. 1224-1 of the French Labor Code, all employment contracts entered into by the Absorbed Company and in force at the Completion Date will survive between the Absorbing Company and the employees of the Absorbed Company after the Merger. |
90
| (d) | Generally, the Absorbing Company will assume all the liabilities of the Absorbed Company, as such liabilities will exist on the Completion Date, including the liabilities that would not have been expressly recognized and transferred pursuant to the Merger Agreement as well as the liabilities having a cause prior to the Completion Date but that would only become apparent after such date. |
| (e) | The Absorbing Company will be required to continue until their expiration or will terminate at its own expense, without recourse against the Absorbed Company, all agreements to which the latter is party. |
| (f) | The Absorbing Company will be subrogated in the benefit of all rights as well as in the benefit and the burden of all contracts, treaties, agreements, markets concluded by the Absorbed Company relating to the contributed assets, with all administrations and all third parties, as well as in the benefit and the burden of all authorizations or administrative permissions which would have been granted to the Absorbed Company. |
| (g) | Similarly, the Absorbing Company will be substituted for the Absorbed Company in all rights and obligations of leases of movable and immovable property held by the Absorbed Company and will pay the corresponding rents, all at its own risk. Consequently, it will be substituted in all the rights and obligations of the leases and agreements. |
| (h) | The Absorbing Company shall have, as from the Completion Date, all powers to bring or continue, in the place and stead of the Absorbed Company, all legal actions and arbitration proceedings, to grant all acquiescence to all decisions, to receive or pay all sums due as a result of such actions, proceedings and decisions. |
| (i) | The Absorbing Company will be required to pay the liabilities of the Absorbed Company to it on the terms and conditions on which they are and will become due, to pay all interest, and generally, to perform all the conditions of any loan agreements and debt instruments that may exist, as the Absorbed Company is required to do, and even with any related prepayments if necessary. |
| (j) | In the event of a difference, plus or minus, between the liabilities set out above and the sums claimed by third parties and recognized as due, the Absorbing Company shall be required to pay or shall benefit from any surplus, without any possible claim on either side. The same shall apply in the event of insufficiency of the provisions included in the liabilities assumed. |
| (k) | The Absorbing Company shall comply with all laws, decrees and orders, regulations and practices relating to the operation of the contributed assets and activities. |
| (l) | The Absorbing Company shall carry out all formalities required to make the transfer of the various assets or rights contributed effective against third parties, all powers being given for this purpose to the bearer of a copy or extract of the present document. |
| (m) | The Absorbed Company shall, at the first request of the Absorbing Company, assist in the drawing up, and provide it with all assistance in this respect, of all additional, amending, reiterating or confirming deeds of this deed and provide all justifications and signatures that may be necessary to carry out the proper transfer of the contributed assets and rights and in particular of the securities and guarantees transferred. |
91
| 12. | UNDERTAKING OF THE PARTIES |
Without prejudice to the provisions of Article 3.8 of the MoU, the Absorbed Company and the Absorbing Company each undertake, until the Completion Date, except with the express prior consent of the other Party (which consent shall not be unreasonably withheld), (i) to continue to operate their respective businesses in the ordinary course of business (ii) to use their best efforts to preserve their assets and business relationships, except for any event occurring in the ordinary course of business.
| 13. | REPRESENTATIONS OF THE PARTIES |
| 13.1 | Representation of the Absorbing Company |
The Absorbing Company represents and warrants to the Absorbed Company, on the date of the Merger Agreement and on the Completion Date:
| (a) | that it has the capacity and has obtained the necessary authorizations from its competent corporate bodies to sign and execute the Merger Agreement; |
| (b) | that it has never been in a state of suspension of payments, has never been the subject of safeguard, recovery or judicial liquidation proceedings, has never been the subject of collective proceedings and, in general, that it has the full capacity to dispose of its rights and property; |
| (c) | that its share capital, on a non-diluted basis and on a fully diluted basis, is composed as of the date of the Merger Agreement as described in Article 2.2 (subject to the Contributions in Kind as described in Article 10.1); |
| (d) | that it is not currently, nor, to its knowledge, likely to be in the future, the subject of any proceedings that could significantly impede or prohibit the exercise of its activity; |
| (e) | it has obtained all contractual, administrative or other authorizations that may be required to validly ensure the completion of the Merger; and |
| (f) | that the New Shares (as this term is defined hereinafter) that it will issue as consideration for the Merger will be issued in full ownership and that they will be free of any restriction, security interest, option, pledge, lien or right of any kind that could restrict the ownership of such New Shares (as this term is defined hereinafter), except for the New Shares that are subject to a retention period in accordance with the provisions of the regulations of the free share allocation plan adopted by the board of directors of the Absorbed Company on May 19, 2022. |
92
| 13.2 | Representation of the Absorbed Company |
The Absorbed Company represents and warrants to the Absorbing Company, on the date of the Merger Agreement and on the Completion Date:
| (g) | that it has the capacity and has obtained the necessary authorizations from its competent corporate bodies to sign and execute the Merger Agreement; |
| (h) | that it has never been in a state of suspension of payments, has never been the subject of safeguard, recovery or judicial liquidation proceedings, has never been the subject of collective proceedings and, in general, that it has the full capacity to dispose of its rights and property; |
| (i) | that its share capital is composed at the date of the Merger Agreement as described in Article 2.1 (subject to the Contributions in Kind as described in Article 10.1); |
| (j) | that it is not currently, nor, to its knowledge, likely to be in the future, the subject of any proceedings that could significantly impede or prohibit the exercise of its activity; |
| (k) | that its assets are not subject to any pledge or lien of such a nature, outside the normal course of business, as to restrict the use or exercise of the right of ownership; |
| (l) | it has obtained, or will obtain at the latest on the Completion Date, all contractual, administrative or other authorizations that may be necessary to validly ensure the completion of the Merger; and |
| (m) | that its assets are not threatened by any expropriation measures. |
| 14. | SHARES EXCHANGE RATIO AND REMUNERATION OF THE CONTRIBUTION |
| 14.1 | Shares exchange report |
In order to determine the remuneration of the Merger of the Absorbed Company, the value of the Absorbed Company and the Absorbing Company, as well as their shares, was assessed according to the methods set forth in Schedule 14.1.
It was retained:
| • | as the value of the Pherecydes Shares, their real value, i.e. a value of EUR 2.29 per Pherecydes Share determined in accordance with the valuation methods described in Schedule 14.1; and |
| • | as the value of the Erytech Shares, their real value, i.e. a value of EUR 0.61 per Erytech Share determined in accordance with the valuation methods described in Schedule 14.1. |
As a result of these respective valuations, the exchange ratio retained in the context of the Merger is fifteen (15) Erytech Shares for four (4) Pherecydes Shares.
93
| 14.2 | Remuneration of the contribution |
In remuneration and representation of the net assets contributed by the Absorbed Company, the shareholders of the Absorbed Company (with the exception of the Absorbed Company with respect to the Treasury Shares and of Erytech with respect to the shares held at the end of the Contributions in Kind, as well as at the Completion Date) are granted 26,575,893 new shares with a nominal value of ten cents of euro (EUR 0.10) each, fully paid up, to be created by the Absorbing Company, according to the exchange ratio mentioned in Article 14.1 (the “New Shares”).
The 26,575,893 New Shares will be subject to all the provisions of the by-laws of the Absorbing Company and will have the same rights as the existing Erytech Shares. They will be created with current dividend rights and will be entitled to the benefit of the Completion Date.
This remuneration has been determined taking into account the application of the valuation methods described in Article 14.1.
In any event, in case of modification of the number of Pherecydes Shares that will be held by the Absorbing Company at the Completion Date and/or of the number of shares composing the share capital of the Absorbed Company by the effect of an interim transaction between the date hereof and the Completion Date, the number of New Shares of the Absorbing Company to be issued as remuneration for the Merger and, accordingly, the nominal amount of the resulting capital increase, will be automatically adjusted accordingly.
| 14.3 | Capital Increase of the Absorbing Company—Amount of the Merger Premium |
| 14.3.1 | No exchange of Treasury Shares and Pherecydes Shares held by the Absorbing Company |
Pursuant to the provisions of Article L. 236-3 II of the French Commercial Code, the Pherecydes Shares held by the Absorbing Company following the Contribution in Kind and on the Completion Date shall not be exchanged, nor shall the Treasury Shares be exchanged, which shares shall be cancelled as of right on the Completion Date.
The Absorbing Company will therefore only increase its capital in order to remunerate the shareholders of the Absorbed Company for the fraction of net assets corresponding to the shares that it does not hold in the Absorbed Company on the Completion Date.
The value of the net assets transferred in respect of the shareholding of the Absorbing Company in the Absorbed Company, and the net book value of the shares of the Absorbed Company to be held by the Absorbing Company having been determined on the same basis and consequently being identical as of today’s date, no merger bonus or loss shall be recognized in the accounts of the Absorbing Company, except for the completion of the transaction between the date hereof and the Completion Date.
94
| 14.3.2 | Capital increase of the Absorbing Company |
In remuneration of the contributions made by the Absorbed Company to the benefit of the Absorbing Company, other than those subject to a merger-waiver, the Absorbing Company will proceed to an increase of its share capital by an amount of EUR 2,657,589.30, by creation of 26,575.893 New Shares with a nominal value of ten cents of euro (EUR 0.10) each, with a balancing payment of a total amount of forty-two cents of euro (EUR 0.42); it being specified that the final number of New Shares to be issued and, accordingly, the nominal amount of the resulting capital increase will be adjusted by operation of law according to the exact number of Pherecydes Shares to be remunerated under the Merger in the event, among other things, that their amount would be adjusted as a result of an interim transaction between the date hereof and the Completion Date.
Without prejudice to Article 14.4, the Absorbing Company will not proceed to any compensation of and the shareholders of the Absorbed Company expressly waive the payment of any balancing of any balancing payment.
The New Shares thus issued will carry dividend rights as from the Completion Date, will be fully assimilated to the existing Erytech Shares of the Absorbing Company and will use the same rights and bear the same expenses, in particular any withholding tax, so that all the Erytech Shares, without distinction, will give the right to the payment of the same net amount at the time of any distribution, apportionment or reimbursement carried out during the term of the Absorbing Company or at the time of its liquidation.
The New Shares will all be negotiable as soon as the capital increase of the Absorbing Company remunerating the Merger is completed, in accordance with article L. 228-10 of the French Commercial Code, and will immediately be the subject of an application for admission to trading on compartment C of the regulated market of Euronext Paris.
It is specified that on the Completion Date, the New Shares will not be registered with the Securities Exchange Commission nor will they be the subject of an application for admission to trading on Nasdaq (and no application will be made in this respect by the Parties).
| 14.3.3 | Expected amount of the merger premium |
| Amount (EUR) | ||||
| The amount of the net assets remunerated by the Absorbing Company amounting to: |
14 757 430.84 | |||
| The amount of the nominal capital increase of the Absorbing Company with a total balancing payment of EUR 0.42 amounting to: |
2 657 589.72 | |||
| The difference represents the Merger Premium, which is estimated at: |
12 099 841.12 | |||
The Merger Premium, on which the rights of the existing and new shareholders of the Absorbing Company shall be based, shall be entered as a liability on the balance sheet of the Absorbing Company. It may be allocated in accordance with the applicable principles decided by the shareholder(s) of this company.
95
In particular, it may be proposed to the shareholders of the Absorbing Company, called to approve the Merger Agreement, to decide or to authorize all deductions from its amount in view of the allocation of all or part of the costs and duties resulting from the Merger and for the reversal of any reserve or regulated provision resulting from the application of the preferential tax regime to which the Merger is subject.
| 14.4 | Fractional shares |
The rights forming fractional shares will not be negotiable or transferable. Consequently, in accordance with the provisions of articles L. 228-6-1 and R. 228-12 of the French Commercial Code, when the number of Erytech Shares to which a Pherecydes shareholder is entitled does not correspond to a whole number of Erytech Shares, the shareholder will receive the number of Erytech Shares immediately below, plus of a cash balance resulting from the price at which the Erytech Shares corresponding to the fractional shares will have been sold by the financial intermediaries, within a period of thirty days as from the latest of the dates of registration, in the account of the Pherecydes shareholders, of the whole number of Erytech Shares allotted.
| 14.5 | Financial instruments giving access to the capital of the Absorbed Company |
Pursuant to articles L. 225-197-1 and L. 228-98 to L. 228-106 of the French Commercial Code and article 6.3.2 of the regulations of the free share allocation plan adopted by the board of directors of the Absorbed Company on May 19, 2022, the Absorbing Company will automatically replace the Absorbed Company in its obligations towards the beneficiaries of free Pherecydes Shares and the beneficiaries of founders warrants (the “BSPCE”).
The rights of the beneficiaries will therefore be transferred to New Shares in application of the merger parity referred to in Article 14.1 according to the following formalities: the number of shares of the Absorbed Company to which each beneficiary would be entitled in the case of the same allocation plan will correspond to the number of shares of the Absorbing Company to which he/she would have been entitled under this plan multiplied by the merger parity applicable to the shareholders referred to in Article 14.1, the number thus obtained being rounded down to the nearest whole number. It is specified that the beneficiaries of BSPCE and free shares will be notified of the above conditions by the Absorbed Company, before the Completion Date.
The other provisions provided for in the regulations and plans for the allocation of free shares and BSPCE, and in particular the provisions relating to the vesting and retention periods, for their remaining duration on the Completion Date, shall remain applicable to the rights and allocation and to the New Shares received in exchange by the beneficiaries.
The extraordinary general meeting of the Absorbing Company called to rule on the Merger will acknowledge of the obligations of the Absorbing Company resulting from this assumption of the commitments of the Absorbed Company and will waive the preferential subscription right to the ordinary shares that will be issued by the Absorbing Company as a result of the exercise of the BSPCE and the definitive allocation of the free shares.
96
| 15. | DISSOLUTION OF THE ABSORBED COMPANY |
In accordance with Article L. 236-3 of the French Commercial Code, the completion of the Merger will result in the dissolution without liquidation of the Absorbed Company and the transfer of all its assets and liabilities to the Absorbing Company.
| 16. | CONDITIONS PRECEDENT TO THE COMPLETION OF THE MERGER AND SUBSEQUENT TRANSACTIONS |
The Merger and the resulting increase in the share capital of the Absorbing Company will only become final subject to and solely as a result of the lifting of the following conditions precedent:
| • | the delivery by the statutory auditor of the Merger of (i) a report on the value of the contributions and (ii) a report on the terms and conditions of the Merger confirming the fairness of the exchange ratio retained; |
| • | the approval by the general shareholders’ meeting of the Absorbed Company of the Merger and the resulting dissolution of the Absorbed Company; |
| • | the approval by the general shareholders’ meeting of the Absorbing Company of the Merger as well as the capital increase allowing the remuneration provided for in Article 14.3; and |
| • | the approval by the general shareholders’ meeting of the Absorbing Company of the resolutions relating to the appointment of the directors appointed by Pherecydes and the amendment of Erytech’s by-laws relating to the removal of the casting vote of the chairman of the board of directors. |
In accordance with the provisions of Article L. 236-3 of the French Commercial Code, the definitive completion date of the Merger and of the capital increase of the Absorbing Company will be the date of completion of the last of the conditions precedent referred to above (the “Completion Date”).
If these conditions are not fulfilled on July 31, 2023 at midnight at the latest, this Merger Agreement will be considered as null and void, without any indemnity on either side, unless both Parties waive it, without prejudice to the provisions of Article 18.8.
| 17. | TAX RETURNS AND OBLIGATIONS |
| 17.1 | General undertaking |
The Parties undertake to comply with all applicable legal provisions relating to the declarations to be made for the payment of corporate income tax and any other tax or duty resulting from the completion of the Merger in the context set forth hereinafter.
97
The Parties represent:
| • | that they are subject to corporate income tax; |
| • | that the contribution of the assets and liabilities of the Absorbed Company will be remunerated by the allocation to the shareholders of the latter of rights representing the capital of the Absorbing Company within the meaning of article 301 F of schedule II to the French General Tax Code; |
| • | with respect to corporate income tax, to place the Merger transaction under the preferential tax regime for mergers as set forth in article 210 A of the French General Tax Code; and |
| • | as far as registration duties are concerned, to place the Merger transaction under the regime provided for in article 816 of the French General Tax Code. |
As a result, the options and commitments relating to the present Merger Agreement are as follows
| 17.2 | Retroactivity |
In accordance with the provisions of Article 5, the Merger shall take effect, from an accounting and tax point of view, on the Effective Date. Therefore, the results, whether profitable or not, generated since this date by the Absorbed Company will be included in the taxable result of the Absorbing Company.
Consequently, the Absorbing Company undertakes to:
| • | file its income tax return and pay the corporate income tax for which it will be liable for the fiscal year ending December 31, 2023, taking into account the results of its own activities and the results of the activities carried out by the Absorbed Company since the Effective Date; |
| • | file, within 45 days as from the Completion Date, an income tax return in the name of the Absorbed Company, mentioning “None”. This declaration will be accompanied by the statement of follow-up of the capital gains on deferred taxation referred to in article 54 septies of the French General Tax Code. |
| 17.3 | Registration fees |
In accordance with articles 635, 1-5° and 816 of the French General Tax Code, the Merger will be registered free of charge within one month following the Completion Date.
Where applicable, the transfer of any title to real estate will, however, be subject to a real estate security contribution at the rate of 0.1% on the market value of the said property, in accordance with articles 879 and 881 K of the same code.
98
| 17.4 | Corporate income tax |
The Parties declare to submit the Merger to the preferential tax regime provided for in article 210 A of the French General Tax Code. As a consequence, the unrealized capital gains on all contributed fixed assets, as well as the provisions (other than those becoming irrelevant), will not be subject to corporate income tax at the level of the Absorbed Company pursuant to the provisions of article 210 A of the French General Tax Code.
In order to benefit from the provisions of article 210 A of the French General Tax Code, the Absorbing Company undertakes to comply with all of the following provisions and requirements referred to in article 210 A of the French General Tax Code and in particular to:
| • | to include in its liabilities the provisions the taxation of which is deferred at the level of the Absorbed Company and which do not become irrelevant as a result of the Merger, as well as the special reserve where this company has recorded the long-term capital gains previously subject to corporate income tax at a reduced rate of 10%, 15%, 18%, 19% or 25%, as well as the reserve where the provisions for price fluctuation have been recorded in application of the sixth paragraph of the 5° of the 1 of article 39 of the French General Tax Code; |
| • | to substitute itself for the Absorbed Company for the reintegration of the results whose recognition had been deferred for the taxation of the latter; |
| • | to calculate the capital gains realized subsequently on the occasion of the disposal of the non-depreciable fixed assets (and of the portfolio securities assimilated thereto pursuant to the provisions of article 210 A, 6. of the French General Tax Code) received at the time of the Merger on the basis of the value that these assets had, from a tax point of view, in the books of the Absorbed Company; |
| • | reintegrate in its profits subject to corporate income tax, under the conditions and within the time limits set forth in d of 3. of article 210 A of the French General Tax Code, the capital gains generated by the Merger on the contribution of depreciable assets. The reintegration of the capital gains is carried out in equal parts over a period of fifteen years for the constructions and the rights relating to the constructions as well as for the plantations and the fittings and improvements of the depreciable land over a period at least equal to this duration; in the other cases, the reintegration is carried out in equal parts over a period of five years. When the total of the net capital gains on buildings, plantations and land improvements exceeds 90% of the overall net capital gain on depreciable items, the reintegration of the capital gains relating to buildings, plantations and land improvements is carried out in equal parts over a period equal to the weighted average depreciation period of these assets. However, the sale of a depreciable asset results in the immediate taxation of the portion of the capital gain relating to this asset that has not yet been reintegrated. On the other hand, the subsequent depreciation and capital gains relating to the depreciable items are calculated on the basis of the value attributed to them at the time of the merger; |
| • | as from the fiscal year during which the Absorbing Company deducts from its taxable income, pursuant to the third paragraph of 2° of 1 of article 39, the depreciation of goodwill recorded in the accounts, this goodwill falls under d of 3 of article 210 A of the French General Tax Code. When it does not give rise to a depreciation deducted from the taxable income, the goodwill received falls under c of 3 of article 210 A of the French General Tax Code; and |
99
| • | record in its balance sheet the non-capitalized items included in the Merger for the value that these items had, from a tax point of view, in the books of the Absorbed Company; failing this, the Absorbing Company shall include in its results of the financial year of the Completion Date the profit corresponding to the difference between the new value of these items and the value that they had, from a tax point of view, in the books of the Absorbed Company. |
The Absorbing Company undertakes, moreover, to attach to its income tax return for as long as necessary a statement of follow-up of the capital gains subject to tax deferral in accordance with the model provided by the administration, showing, for each type of item, the information necessary for the calculation of the taxable result of the subsequent disposal of the items in question, in accordance with article 54 septies I of the French General Tax Code and article 38 quindecies of annex III to the same Code.
The Absorbing Company also undertakes to attach the said statement of follow-up of the capital gains subject to tax deferral to the income tax return of the year of termination of the Absorbed Company provided for in article 201 of the French General Tax Code, which must be filed within sixty (60) days of the first publication of the Merger in a legal listing support.
The Absorbing Company will record for as long as necessary the capital gains generated on the non-depreciable assets, the taxation of which has been deferred, in the register provided for in article 54 septies II of the French General Tax Code.
| 17.5 | Value Added Tax |
At the time of the Merger, each of the Parties being taxable persons liable for VAT, the supplies of goods, services and operations mentioned in the 6th and 7th articles of the French General Tax Code will be exempted from VAT, pursuant to the article 257 bis of the French General Tax Code, as commented by the BOFiP (BOI-TVA-DED-60-20-10 and BOI-TVA-CHAMP-10-50-10).
In accordance with paragraph 280 of the BOFiP BOI-TVA-DED-60-20-10, the Absorbing Company, insofar as it is deemed to continue the person of the Absorbed Company, will have to make, if necessary the adjustments of the right to deduction provided for in articles 206 and 207 of the schedule II to the French General Tax Code and the taxes of transfers or self-supplies which would become due after the Merger and which would have been in principle incumbent on the Absorbed Company if the latter had continued to exploit itself the transferred universe.
In accordance with the BOFiP BOI-TVA-CHAMP-10-10-50-10, the Absorbed Company declares to transfer purely and simply to the Absorbing Company, which will thus be subrogated in all its rights and obligations, the VAT credit which it would have at the Date of Realization.
100
Each of the Parties declares that the amount, exclusive of tax, of the supplies of goods and services realized within the framework of the Merger will be entered on their respective CA3 turnover declarations filed for the period during which the transaction was realized, on line E2 “other non-taxable transactions”.
| 17.6 | Previous transactions |
The Absorbing Company declares that it will take over the benefit or the burden of all the tax undertakings that could have been previously subscribed by the Absorbed Company, and of all the approvals that could have been granted to it.
| 18. | MISCELLANEOUS |
| 18.1 | Delivery of titles |
Once the Merger Agreement has become final, all property titles, deeds, documents and other materials relating to the contributed assets and rights will be delivered to the Absorbing Company.
| 18.2 | Formalities |
The Parties shall complete, within the legal deadlines, all legal formalities of publication and filing relating to the Merger.
The Merger Agreement will be published in accordance with the law and in such a way that the period granted to the corporate creditors to file an objection following such publication will expire before the general shareholders’ meetings of the Absorbed Company and the Absorbing Company convened to decide on the Merger Agreement.
Any objections will be brought before the competent Commercial Court, which will settle them.
This Merger Agreement will be registered with the tax authorities free of charge.
| 18.3 | Powers |
All powers are given to the bearer of an original, a copy or an extract of the present document to carry out all filings or publications prescribed by law, in particular with a view to the running of the period granted to the creditors and, more generally, to carry out all legal formalities and make all notifications that may be necessary.
| 18.4 | Fees |
The costs and duties of the Merger Agreement, and all those which will be the direct or indirect consequence thereof, will be borne by the Absorbing Company as capital increase costs.
101
| 18.5 | Election of domicile |
For the execution of the Merger Agreement, the undersigned elect domicile at the registered offices of the companies they represent.
| 18.6 | Amendment—Waiver—Enforcement |
| (n) | Any alteration, modification or amendment to the provisions of the Merger Agreement will require a written agreement validly signed by all the Parties. The Parties declare that they assume, each as far as it is concerned, the risk of occurrence, until the Completion Date, of a change of circumstances unforeseeable at the time of the conclusion of the Merger Agreement and thus waive the provisions of article 1195 of the civil code in such a case. As the case may be, each of the Parties acknowledges that, as of the date of the Merger Agreement, there are no circumstances that could make the execution of the Merger Agreement excessively onerous. |
| (o) | No waiver of any provision or condition of the Merger Agreement, nor any consent required under the Merger Agreement, shall be validly made without a written statement signed by the waiving or consenting Party and only to the extent of such statement. |
| (p) | In the absence of a time limit specifically provided in the Merger Agreement to exercise or waive a right, the failure to exercise such right or any act that could be construed as a waiver of such right but not formalized in writing shall in no event be deemed or construed as final. |
| (q) | The Parties undertake to communicate, to sign and to deliver any information and any document as well as to perform any act or take any decision that may be necessary for the execution of the Merger Agreement. |
| 18.7 | Autonomy of stipulations |
In the event that any provision of the Merger Agreement is declared void or ineffective for any reason whatsoever, the application of the other provisions of the Merger Agreement shall not be affected. In such case, the Parties undertake to negotiate in good faith in order to substitute such provision with a valid provision giving as much effect as possible to the intention of the Parties.
| 18.8 | Entire agreement, reiteration of MoU stipulations |
Subject to the following, the Merger Agreement constitutes the entire and sole agreement between the Parties with respect to the rules of transfer of the assets and liabilities of the Absorbed Company and supersedes any prior agreement, whether oral or written.
The Parties agree to reiterate the provisions of the following articles of the MoU: 1, 2.3, 3.1, 3.3, 3.4, 3.5.2, 3.6 to 3.13, 4.3 and 5, so that these provisions shall remain applicable until the Completion Date, with the exception of articles 4.3 and 5 of the MoU, which shall continue to apply in case of lapse or termination of this Merger Agreement.
102
| 18.9 | Freely negotiated agreement |
Each of the Parties acknowledges that it was free to assess and negotiate the terms and conditions of the Merger Agreement. Consequently, each of the Parties acknowledges that the Merger Agreement does not constitute a adhesion agreement in the sense of Article 1110 of the French Civil Code.
| 18.10 | Interdependent agreements |
The Parties expressly waive any right they may have pursuant to Article 1186 of the French Civil Code, to rely on the lapse of the Merger Agreement due to the disappearance, for any reason whatsoever, of any other contract necessary for the realization of the operations subject to the Merger Agreement.
| 18.11 | Applicable law |
This Merger Agreement is subject to French law.
| 18.12 | Jurisdiction |
Any dispute or litigation relating to the validity, interpretation, performance or termination of the Merger Agreement shall be submitted to the exclusive jurisdiction of the Commercial Court of Paris.
| 18.13 | Electronic signature |
In accordance with Articles 1366 and 1367 of the French Civil Code, the Merger Agreement is signed electronically by the respective authorized representatives of the Parties mentioned in the above appearances. The Parties expressly acknowledge that electronic signatures via DocuSign have been used for the signature of the Merger Agreement by these signatories. Each Party acknowledges that it has received all the information required for the electronic signature of the Merger Agreement and that it has signed the Merger Agreement electronically with full knowledge of the technology used and its terms and conditions, and therefore waives any claim or legal action to challenge the reliability of such electronic signature system or its intention to enter into the Merger Agreement. Furthermore, in accordance with the provisions of Article 1375 of the French Civil Code, the obligation to deliver an original paper copy to each of the Parties is not necessary as evidence of the commitments and obligations of each Party to this agreement. The delivery of an electronic copy of the Merger Agreement directly by Docusign to each of the Parties constitutes sufficient and irrefutable proof of the commitments and obligations of the latter.
103
SIGNATURES
Signed by electronic signature,
May 15, 2023,
| For Erytech
Gil Beyen |
||
| General Manager |
| For Pherecydes
Thibaut du Fayet |
||
| General Manager |
104
LIST OF SCHEDULES
| Schedule 2.1.2 | Summary of financial instruments giving access to the capital of the Absorbed Company | |
| Schedule 2.2.2 | Summary of financial instruments giving access to the capital of the Absorbing Company | |
| Schedule 6 | Reference Accounts of the Absorbed Company | |
| Schedule 10.1 | List of Investors in Guy Rigaud Pool | |
| Schedule 14.1 | Valuation methods of the fair value of the Absorbed Company and the Absorbing Company and remuneration of the Merger | |
105
SCHEDULE 2.1.22.2.1
SUMMARY OF THE FINANCIAL INSTRUMENTS GIVING ACCESS TO THE CAPITAL
OF THE ABSORBED COMPANY
106
| BSPCE 2017 | BSPCE 2019-1 | BSPCE 2019-2 | BSPCE 2019-4 | BSPCE 2020-1 | BSPCE 2020-2 | BSPCE 2020-3 | BSPCE 2021-1 | BSPCE 2021-2 | BSPCE 2021-3 | BSPCE 2021-4 | Total | |||||||||||||||||||||||||||||||||||||
| Date of meeting |
12/22/2017 | 06/28/2019 | 06/28/2019 | 06/28/2019 | 05/28/2020 | 05/28/2020 | 05/28/2020 | 12/24/2020 | 12/24/2020 | 12/24/2020 | 12/24/2020 | N/A | ||||||||||||||||||||||||||||||||||||
| Date of decision by the Board of Directors |
03/22/2018 | 09/12/2019 | 11/28/2019 | 06/24/2020 | 06/19/2020 | 06/19/2020 | 06/19/2020 | 02/04/2021 | 02/04/2021 | 07/07/2021 | 11/29/2021 | N/A | ||||||||||||||||||||||||||||||||||||
| Total number of BSPCE authorized |
171,500 | 116,650 | (2) | 116,650 | (2) | 116,650 | (2) | 263,000 | (3) (4) | 263,000 | (3) (4 | 263,000 | (3) (4) | See | (3) | See | (3) | See | (3) | See | (3) | N/A | ||||||||||||||||||||||||||
| Total number of BSPCE granted |
59,850 | 28,350 | 20,000 | 700 | 106,500 | 129,000 | 27,500 | 273,500 | 20,000 | 18,000 | 233,000 | 916,400 | ||||||||||||||||||||||||||||||||||||
| Total number of ordinary shares of the Absorbed Company that may be subscribed for at the date of grant |
59,850 | 28,350 | 20,000 | 700 | 106,500 | 129,000 | 27,500 | 273,500 | 20,000 | 18,000 | 233,000 | 916,400 | ||||||||||||||||||||||||||||||||||||
| Starting point for the exercise of the BSPCE |
03/22/2020 | (6) | 09/12/2020 | (7) | 11/28/2020 | (8) | 06/24/2021 | (9) | 06/19/2021 | (10) | 06/19/2020 | (11) | 06/19/2020 | (12) | 02/04/2021 | 02/04/2021 | (13) | 07/07/2021 | 11/29/2021 | N/A | ||||||||||||||||||||||||||||
| Expiration date of the BSPCEs |
46,833 | 47,372 | 47,449 | 47,657 | 47,652 | 47,652 | 47,652 | 47,882 | 47,882 | 48,035 | 48,180 | 524,246 | ||||||||||||||||||||||||||||||||||||
| Issue price of a BSPCE |
5.420 | 4.070 | 3.450 | 4.070 | 2.035 | 2.035 | 2.035 | 6.000 | 6.000 | 8.200 | 7.090 | N/A | ||||||||||||||||||||||||||||||||||||
| Number of common shares subscribed for as of May 5, 2023 |
350 | 1,049 | 0 | 0 | 18,778 | 43,776 | 0 | 1,773 | 0 | 0 | 0 | 65,726 | ||||||||||||||||||||||||||||||||||||
| Total number of BSPCE cancelled or expired as of May 5, 2023 |
55,300 | 17,763 | 0 | 0 | 31,722 | 15,224 | 10,000 | 64,000 | 0 | 1,125 | 66,800 | 261,934 | ||||||||||||||||||||||||||||||||||||
| Total number of BSPCE outstanding as of May 5, 2023 |
4,200 | 9,538 | 20,000 | 700 | 56,000 | 70,000 | 17,500 | 207,727 | 20,000 | 16,875 | 166,200 | 588,740 | ||||||||||||||||||||||||||||||||||||
| Total number of BSPCE outstanding as of May 5, 2023 and exercisable |
4,200 | 6,911 | 15,000 | 350 | 36,450 | 70,000 | 17,500 | 207,727 | 15,000 | 8,625 | 114,180 | 495,943 | ||||||||||||||||||||||||||||||||||||
| Total number of ordinary shares that may be subscribed upon exercise of the BSPCEs outstanding as of May 5, 2023 (including BSPCEs not exercisable as of May 5, 2023) |
4,200 | 9,538 | 20,000 | 700 | 56,000 | 70,000 | 17,500 | 207,727 | 20,000 | 16,875 | 166,200 | 588,740 | ||||||||||||||||||||||||||||||||||||
| Maximum total number of ordinary shares of the Absorbed Company that may be subscribed upon exercise of the outstanding BSPCEs (assuming the fulfillment of all the conditions for the exercise of said BSPCEs) |
4,200 | 9,538 | 20,000 | 700 | 56,000 | 70,000 | 17,500 | 207,727 | 20,000 | 16,875 | 166,200 | 588,740 | ||||||||||||||||||||||||||||||||||||
| Maximum total number of ordinary shares of the Absorbing Company that may be subscribed for upon exercise of the outstanding BSPCEs (assuming the completion of the merger and all of the conditions for the exercise of the said BSPCEs) at the Completion Date) |
15,749 | 35,765 | 75,000 | 2,625 | 210,000 | 262,500 | 65,625 | 778,976 | 75,000 | 63,281 | 623,250 | 2,207,771 | ||||||||||||||||||||||||||||||||||||
It is specified that the Merger will not result in the early vesting of any BSPCE plan
| (1) | The BSPCE were granted by the Annual General Meeting of June 28, 2016 |
| (2) | The 2019-1, 2019-2, 2019-3 and 2019-4 BSPCEs were authorized by the General Meeting of June 28, 2019, authorizing the issue of a maximum of 116,650 BSPCEs |
| (3) | The BSPCE 2020-1, 2020-2 and 2020-3 were authorized by the General Meeting of May 28, 2020, authorizing the issue of a maximum of 263,000 BSPCE |
| (4) | Under the terms of the minutes of the General Meeting of December 24, 2020, a number of BSPCEs may be granted representing a maximum of 10% of the shares comprising the share capital of Pherecydes on the date of grant |
| (5) | The 2016 BSPCEs may be exercised in tranches representing 1/3 of the total number of BSPCEs granted, starting on January 4, 2017, 2018 and 2019 respectively |
| (6) | 50% of the 2017 BSPCEs may be exercised subject to the beneficiary’s presence in the Company’s active workforce two years after issue, and 50% may be exercised subject to the Company achieving scientific results |
| (7) | The 2019-1 BSPCEs may be exercised in tranches representing 1/4 of the total number of BSPCEs granted, starting on September 11, 2020, 2021, 2022 and 2023 respectively |
| (8) | The 2019-2 BSPCEs may be exercised in tranches representing 1/4 of the total number of BSPCEs granted, starting on November 28, 2020, 2021, 2022 and 2023 respectively |
| (9) | The 2019-4 BSPCEs may be exercised in tranches representing 1/4 of the total number of BSPCEs granted, starting on June 24, 2021, 2022, 2023 and 2024 respectively |
| (10) | The 2020-1 BSPCEs may be exercised in tranches representing 1/3 of the total number of BSPCEs granted, starting on June 19, 2021, 2022, and 2023 respectively |
| (11) | BSPCEs may be exercised subject to the beneficiary’s presence on the exercise date and the achievement of financial objectives |
| (12) | BSPCEs may be exercised subject to the beneficiary’s presence on the exercise date and the achievement of financial objectives |
107
| Free shares (AGA) | ||
| Date of meeting | 19/05/2022 | |
| Date of decision of the Board of Directors | 19/05/2022 | |
| Total number of AGAs granted | 58 523 | |
| Total number of ordinary shares of the Absorbed Company that may be subscribed for at the date of grant | 58 523 | |
| Starting point of the AGM exercise | 19/05/2023 | |
| Acquisition periods (1) | 25% of the AGAs will vest on May 19, 2023; 25% of the AGAs will vest on May 19, 2024; 25% of the AGMs will vest on May 19, 2025. The balance will vest on May 29, 2026. | |
| Conservation period | Retention period of 1 year at the end of the acquisition period. | |
| Number of AGAs under retention period | 0 | |
| Applicable protective measures | If, during the Vesting and Retention Periods, the Absorbed Company carries out a transaction referred to in article 225-197-1 III of the French Commercial Code, the Beneficiary will be able to exercise his rights in the company resulting from the merger, and the remaining term of each Vesting or Retention Period will remain applicable to the allocation rights, or, as the case may be, to the shares received in exchange and the provisions of the By-Laws will remain applicable mutatis mutandis. | |
| Parity adjustment | The new number of shares likely to be allocated to the Beneficiary at the end of each Vesting Period will be determined by correcting the number of shares that are expected to be issued prior to the beginning of the relevant transaction by the exchange ratio of the shares against the shares of the Absorbing Company. | |
| Maximum total number of ordinary shares of the Absorbed Company to which the AGAs granted entitle the holder | 58 523 (2) | |
| Maximum total number of ordinary shares of the Absorbing Company to which the AGAs granted by the Absorbed Company entitle (assuming completion of the Merger) | 219 461 | |
| (1) | The Merger is not likely to trigger an acceleration of the Vesting Period or the Retention Period of the AGAs, thus affecting the allocation, vesting and retention schedule set forth in the table. |
| (2) | 25% of the AGAs, i.e. 14,630 AGAs, will be acquired on May 19, 2023, i.e. before the Merger Completion Date, by the sole beneficiary of the AGAs, Mr. Thibaut Du Fayet. Pursuant to the exchange ratio, 14,630 AGAs issued by the Absorbed Company will give right, as from the Completion Date of the Merger, to 54,863 AGAs in the Absorbing Company, subject to a Retention Period valid until May 19, 2024. |
108
SCHEDULE 2.2.2
SUMMARY OF THE FINANCIAL INSTRUMENTS GIVING ACCESS TO THE CAPITAL OF THE ABSORBING COMPANY
109
| 12/31/2022 | ||||||||||||||||||||||||||||||||
| Non diluted | Diluted* | |||||||||||||||||||||||||||||||
| SHAREHOLDERS |
SHARES | % of capital |
VOTING RIGHTS |
% of total voting rights |
SHARES | % of capital |
VOTING RIGHTS |
% of total voting rights |
||||||||||||||||||||||||
| MANAGEMENT |
27 248 | 0,09 | % | |
38 421 |
|
0,12 | % | 1 638 643 | 4,25 | % | 1 649 816 | 4,12 | % | ||||||||||||||||||
| Gil BEYEN |
4 840 | 0,02 | % | |
7 308 |
|
0,02 | % | 500 986 | 1,30 | % | 503 454 | 1,26 | % | ||||||||||||||||||
| Eric SOYER |
6 264 | 0,02 | % | |
8 574 |
|
0,03 | % | 190 340 | 0,49 | % | 192 650 | 0,48 | % | ||||||||||||||||||
| Jérôme BAILLY |
3 798 | 0,01 | % | |
5 619 |
|
0,02 | % | 109 837 | 0,29 | % | 111 658 | 0,28 | % | ||||||||||||||||||
| Others in management |
12 346 | 0,04 | % | |
16 920 |
|
0,05 | % | 837 480 | 2,17 | % | 842 054 | 2,10 | % | ||||||||||||||||||
| AURIGA Partners |
1 018 212 | 3,28 | % | |
2 036 424 |
|
6,26 | % | 1 018 212 | 2,64 | % | 2 036 424 | 5,09 | % | ||||||||||||||||||
| RECORDATI ORPHAN DRUGS |
431 034 | 1,39 | % | |
862 068 |
|
2,65 | % | 431 034 | 1,12 | % | 862 068 | 2,15 | % | ||||||||||||||||||
| MEMBERS OF THE BoD |
10 303 | 0,03 | % | |
20 606 |
|
0,06 | % | 173 676 | 0,45 | % | 183 979 | 0,46 | % | ||||||||||||||||||
| OTHER SHAREHOLDERS |
42 102 | 0,14 | % | |
67 652 |
|
0,21 | % | 72 102 | 0,19 | % | 97 652 | 0,24 | % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| NOMINATIVE SUB-TOTAL |
1 528 899 | 4,93 | % | |
3 025 171 |
|
9,30 | % | 3 333 667 | 8,65 | % | 4 829 939 | 12,07 | % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Treasury shares |
2 500 | 0,01 | % | — | — | % | 2 500 | 0,01 | % | — | — | % | ||||||||||||||||||||
| BVF Partners L.P. |
97 338 | 0,31 | % | |
97 338 |
|
0,30 | % | 97 338 | 0,25 | % | 97 338 | 0,24 | % | ||||||||||||||||||
| Floating |
29 389 816 | 94,75 | % | |
29 389 816 |
|
90,40 | % | 35 105 119 | 91,09 | % | 35 105 119 | 87,69 | % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| HOLDER SUB-TOTAL |
29 489 654 | 95,07 | % | |
29 487 154 |
|
90,70 | % | 35 204 957 | 91,35 | % | 35 202 457 | 87,93 | % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| TOTAL |
31 018 553 | 100,00 | % | |
32 512 325 |
|
100,00 | % | 38 538 624 | 100,00 | % | 40 032 396 | 100,00 | % | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| * The calculations are made under the hypothesis that: |
| |||||||||||||||
| Amount | Exercise Price |
Expiry | ||||||||||||||
| (i) the exercise of all warrants to subscribe for shares (BSA). warrants to subscribe for business creators’ shares (BSCPE) and stock options. and the definitive acquisition of all bonus shares issued to employees. directors and corporate officers |
|
1 804 768 |
|
|
Cf tab Equity plans |
|
|
Cf tab Equity plans |
|
|||||||
| (ii) the exercise of the warrants (BSA) issued under the OCABSA contract in favor of the Luxembourg fund European High Growth Opportunities Securitization Fund |
|
303 030 |
|
8.91 | 06/25/2025 | |||||||||||
| (iii) the exercise of the warrants (BSA) issued in April 2021 |
|
3 103 449 |
|
7.5 | 05/04/2023 | |||||||||||
| (iv) the exercise of the warrants (BSA) issued in December 2021 |
|
2 308 824 |
|
2.83 | 12/20/2023 | |||||||||||
| Total |
|
7 520 071 |
|
|||||||||||||
110

As of December 31, 2022 we have issued eight. types of share warrants as follows: Plan title BSA 2021 BSA 2020 BSA 2019 BSA 2018 Meeting date June 25, 2021 June 26, 2020 June 21, 2019 June 28, 2018 Dates of allocation July 27, 2021 July 28,2020 October 9, 2019 April 12, 2019 Total Number of BSA authorized 100,000 100,000 200,000 50,000 Total number of BSAs granted 75,250 15,000 75,000 25,998 Start date for the exercise of the BSAs July 27, 2023 July 28, 2022 October9, 2021 One third as from 12A 2020, one third as from 12 April 2021 and one third as from 12 April 2022 BSA expiry date ate July 27,2024 15,000 BSA 2020 have been declared lapsed on 4 November 2020 by the Board of Directors 75,000 BSA 2019 have been declared lapsed on 31 October 2022 by the Board of Directors 25,998 BSA 2018 have been declared lapsed on October 9, 2019 by the Board of Derector BSA exercise price per share €3.82 €6.97 €3.71 €6.52 Number of shares subscribed as of December 31, 2022 0 0 0 0 Total number of BSAs granted but not exercised as of December 31, 2022 13,500 0 0 0 Total number of shares available for subscriptions as of December 31, 2022 0 0 0 0 Maximum number of new shares that can be issued 13,500 0 0 BSA Expired (caducity) 61,750 15,000 75,000 25,998 Plan title BSA 2017 BSA 2016 BSA 2014 BSA 2012 Meeting date June 27,2017 June 24,2016 April 2,2013 May 21,2012 Date of allocation June 27,2017 January 7, 2018 January 8, 2017 December 4,2014 June 23,2015 May 31,2012 August 3,2012 July 18, 2013 July 17, 2014 April 29, 2015 August 31, 2015 Total number of BSAs authorized 100,000 60,000 3,000(i) 11,263 Total number of BSAs granted 95,500 60,000 3,000 Start date for the exercise of the BSAs All BSA 2017 are exercisable since 7 January 2021 All BSA 2016 are exercisable since 8 January 2020 One-third vested in 2015 and two-third vested in 2016 for senior management From May to July 2012, 2013,2014 and 2015 BSA expiry date (3) October 3, 2021 January 22, 2024 May 20,2020 BSA exercise price per share (4) (2) €12.21 €7.36 Number of shares subscribed as of December 31, 2022 0 0 1,000 67,420 Total number of BSAs granted but not exercised as of December 31, 2022 33,750 0 2,900 0 Total number of shares available for subscriptions as December 31, 2022 33,750 0 29,000 0 Maximum number of new shares that can be issued 33,750 0 29,000 0 BSA Expired (caducity) 61750(5) 60,000 0 4,018 SOP As of December 31.2022, the following options have been granted: Date of grant Denomination of the SOP SOP Competent body that granted the SOP Competent body granted the Beneficiars Number of SOP granted Exercise Price Number of sheres that can be subscribed as of December 31,2022 SOP 2016 (Under the 2016 Stock Option Plan) October 3.2016 SOP2016-03102016 Board of Directors Execution Officers 21,999 €18.52 21.399 October 3, 2016 SOP2016-03102016 Chief Executive Officer Employees 22500 €18.52 3,000 January 8,2017 SOP2016-08012017 Chief Executive Officer Employees 3,000 €15.65 June 27,2017 SOP2016-27062017 Chief Executive Officer Employees 18,000 €26.47 9,000 October 3, 2017 SOP2016-03102017 Chief Executive Officer Employees 30,000 €23.59 SOP 2017 undar the 2017 Stock Option Plan; June 27, 2017 SOP2017-27062017 Board of Directors Exexutive officers 12,000 €26.47 12,000 June 27,2017 SOP2017-27062017 Chief Executive Officer Employees 10200 €26.47 1,200 January 7,2018 SOP2017-07012018 Board of DirectorsExecutive Officer 40,500 €18.00 20,250 January 7, 2018 SOP2017-07012018 Chief Executive Officer Employees 56,703 €18.00 8,100 SOP 2018 under the 2018 Stock Option Plan September 7, 2018 SOP2018-07092018 Board ofDirectors Employees 24,000 €9.26 January 6, 2019 SOP2018-06012019 Chief Executive Officer Employees 38,025 €6.38 4,875 April. 12. 2019 SOP2018-12042019 Chief Executive Officer Executive Officers 44,200 €7.20 31,200 April 12,2019 SOP2018-12042019 Chief Executive Officer Employees 32,705 €7.20 5,200 SOP 2019under the 2019 Stock Option Plan July 31.2019 SOP2019-31092019 Board of Directors Excutive officers 59,123 €5.78 59,123 October 9. 2019 9 SOPSOP2019-09102019 Board of DirectorsEmployees 217,500 €4.25 180,000 October 9. 2019 SOP2019-09102019 Board of Directors Employees 129,750 €4.25 210,000 February 25, 2020 SOP2019-25022020 Chief Executive Officer Employees 41,950 €5-87 12,400 SOP2020 under the 2020 Stock Option Plan; July 28,2020 SOP2020-28072020 Board of DirectorsExecutive Officers 247,500 €6.88 210,000 July 28,2020 SOP2020-28072020 Board of Directors Employees 126,500 €6.88 33,000 November 13,,2020 SOP2020-13112020 Chief Executive Officer Executive Officers 75,000 €6.14 June 4, 2021 SOP2020-04062021 Chief Executive Officer Employees 57,000 €4.78 21,500 SOP 2021 under the 2021 Stock Option Plan July 27,2021 SOP2021-27072021 Board of Directors Executive Officers 256,500 €3.71 189,000 July 27,2021 SOP2021-27072021 Board of Directors Employees 121,050 €3.71 37,350 Deramber 16.2021 SOP2021-16122021 Chief Executive Officer Executive Officers 66,000 €2.14 51,000 December 16 2021 , SOP2021-16122021 Chief Executive Officer Employees 83,000 €2.14 44,500 BSPCE We have issued two types of founder’s share warrants as follows : Plan Title BSPCE 2014 BSPCE 2012 Meeting da” April 1, 2013 May 21,2012 Dates of allocation January 22,2014 May 31.2012 June23,2015 July 18,2013 May 6,2016 July 17,2014 Total number of BSPCEs authorized 19,500(1) 33,737 Total numb of BSPCEs granted 18,410(2) 33,787(3) Start date for the exercise of the For senior management one-third was BSPCEs Vested in 2015 and two-thirds were Vested in 2016; for other employees From May to July 2012.2013 and 2014 immediately upon each grant except for 6,500 BSPCE2014 which could not be exercise before July 1,2017 BSPCE expiry date January 22,2024 May 20,2020 BSPCE exercise price per shere €12.250 €7.362 Number of shares- subscribed as of 15,000 184,190 December 31,2022 Total number of BSPCEs granted but 16,910 not exercise as of December 31, 2022 Total number of shares available for 169,100 subscription as of December 31, 2022 Maximum number of new shares that 169,100 can be issue d
111
SCHEDULE 6
REFERENCE ACCOUNTS OF THE ABSORBED COMPANY
(See p. 68 to 72 of the 2022 annual financial report)
112
SCHEDULE 10.1
LIST OF INVESTORS IN THE GUY RIGAUD POOL
113
GUY RIGAUD’S POOL
| HELEA, a French simplified joint stock company, whose registered office is located at 28 Cours de Verdun, 69002 Lyon, registered in the Lyon Trade and Companies Register under number 349 296 194. |
| SOFIDU, a French one-person simplified joint stock company, whose registered office is located at 30 rue Saint Mathieu, 69008 Lyon, registered in the Lyon Trade and Companies Register under number 823 922 232. |
| FINANCIERE SAINT ROMAIN, a French non-trading company, whose registered office is located at 33 rue St Romain 69008 LYON, registered in the Lyon Trade and Companies Register under number 810 131 706. |
| MYROPOLA, a French simplified joint stock company, whose registered office is located at 36 Chemin de Genas, 69800 Saint Priest, registered in the Lyon Trade and Companies Register under number 438 247 124. |
| PAMINOVE, a French limited liability company with a single shareholder, whose registered office is located at 61 Chemin du Moulin Carron, 69570 Dardilly, registered in the Lyon Trade and Companies Register under number 752 634 766. |
| L’ERMIGAUD, a French limited liability company, whose registered office is located at 2 Chemin des Garennes, 69450 Saint Cyr au Mont d’Or, registered in the Lyon Trade and Companies Register under number 753 833 003. |
| Mister Jacques TCHENG, born on 25/04/1948 in Lyon, of French nationality, living at 1 rue Eymard Duvernay, 38700 La Tronche. |
| SOLYS, a French limited liability company, whose registered office is located at 6 place Rouville, 69001 Lyon, registered in the Lyon Trade and Companies Register under number 831 098 587. |
| Miss Valentine GOUEDARD COMTE, born on 17/11/1956, of French nationality, living at 2 place Gensoul, 69002 Lyon. |
| Mister Bernard LINAGE, born on 23/12/1942, of French nationality, living at 51 bis rue de Chazière, 69004 Lyon. |
| M.P DELOCHE & Associés, A French joint-stock company , whose registered office is located at 136 Cours Lafayette, 69003 Lyon, registered in the Lyon Trade and Companies Register under number 491 282 125. |
| SC ROC DE LOU, a French non-trading company with a capital of 1,488,100 euros, whose registered office is located at 44 Rue Victor Hugo, 69002 Lyon, registered in the Lyon Trade and Companies Register under number 831 768 098. |
114
SCHEDULE 14.1
METHODS OF VALUATION OF THE REAL VALUE OF THE MERGED COMPANY AND OF THE ABSORBING COMPANY AND MERGER REMUNERATION
115
| I. | Valuation of the company Erytech Pharma |
| 1. | Principal valuation methods used |
| (A) | Analysis of Erytech Pharma’s share price |
| Share price |
At 01/19/2023 (in € per share) | |||
| Spot |
0.61 | |||
| Average 20 days |
0.49 | |||
| Average 60 days |
0.51 | |||
| Average 120 days |
0.61 | |||
| Average 250 days |
1.01 | |||
| Max. share price 250 days (02/16/2022) |
1.83 | |||
| Min. share price 250 days (12/29/2022) |
0.34 | |||
The analysis of Erytech Pharma’s share price is performed as of January 19, 2023, which corresponds to the date of signature of the LOI on the Merger. The average of the volume weighted prices of the Erytech Pharma share over the last 60 days from January 19, 2023, is equal to 0.51 € per Erytech Pharma share out of a total of 31,016,053 shares of this company. This average results in a valuation of the company of approximately 15.8 M€.
For information, on the eve of the signature of the agreement, the analysis of the Erytech Pharma share confirms the speculative character of the value with volumes higher than those observed historically for this company.
| Analysis of the liquidity of the Erytech Pharma share on 05/12/2023 |
||||||||||||||||
| Securities traded (daily average) |
Average volumes* % total |
Exchanged securities (cumulative) |
Cumulative volumes* % total |
|||||||||||||
| Spot |
419,250 | 1,35 | % | 419,250 | 1.35 | % | ||||||||||
| 20 days |
461,761 | 1,49 | % | 9,235,210 | 29.77 | % | ||||||||||
| 60 days |
295,209 | 0,95 | % | 17,712,520 | 57.10 | % | ||||||||||
| 120 days |
485,966 | 1,57 | % | 58,315,930 | 188.00 | % | ||||||||||
| 250 days |
319,000 | 1,03 | % | 79,750,000 | 257.10 | % | ||||||||||
| 500 days |
233,164 | 0,75 | % | 116,582,200 | 375.85 | % | ||||||||||
| * | Ratio between the number of shares exchanged and the total number of shares issued at that date (i.e. 31,018,553 shares). |
116
Erytech Pharma’s share price over the last 500 trading days, as well as the volume of shares traded over this period are shown in the graph below, for illustrative purposes:

| (B) | Discounted free cash flow (DCF) |
Methodology:
| • | The DCF analysis is based on Erytech Pharma’s 2023e -2025e standalone business plan prepared by the management in view of the search for an industrial partner. |
| • | In the absence of a combination with a new industrial partner, the company’s cost structure raises the question of its ability to continue its operations in the short term, with the free cash flow (DCF) analysis leading to a nil value for the company. Indeed, in the absence of operating income and in view of the personnel costs and the listing in the United States in particular, the DCF method results in a negative value. |
| • | The business plan has therefore been restated to obtain a standalone value allowing the company’s continuity of operation. The main restatements consisted of a cost reduction from 2023e to simulate an operative continuity with 3 FTEs, responsible for maintaining operations from 2025 and beyond. |
Main hypothesis:
| • | The valuation date is 01/01/2023. |
| • | Total number of shares: 31,016,053 (it is specified that there are no shares to be issued in the money at this date). |
| • | The weighted average cost of capital (WACC) used is 12%, reflecting the low risk due to the company’s lack of activity despite its positioning in the biotech sector, where the average cost of capital is between 15% and 20%. |
117
| • | The sum of the free cash flows between 2023-2025ee discounted amounts to -8.5 M€. In view of the company’s net cash position (+24.5 M€), no terminal value has been included, as it is estimated that the reduction in costs would allow the company to continue its operations over a sufficiently long period of time to enable it to find a partner likely to be interested in its know-how, its available cash, or access to the financial markets through its listing on Euronext. |
| • | The enterprise value as of January 1, 2023 is therefore -8.5 M€. After adding the restated net cash position, the value of the shares is €16.2 million as of January 1, 2023. |
On this basis, the free cash flow method shows a center value of €0.52 per Erytech Pharma share.
| 2. | Valuation methods used for information purposes |
| (A) | Analysis of Erytech Pharma’s share price |
| Share price |
As of 02/15/2023 (in € per share) | |||
| Spot |
0.88 | |||
| Average 20 days |
0.92 | |||
| Average 60 days |
0.74 | |||
| Average 120 days |
0.74 | |||
| Average 250 days |
0.97 | |||
| Max. share price 250 days (02/16/2022) |
1.74 | |||
| Min. share price 250 days (12/29/2022) |
0.34 | |||
For information purposes, the average volume-weighted share price of Erytech Pharma over the last 60 days preceding the signature of the MoU on February 15, 2023 is equal to €0.74.
| (B) | Analysts’ price targets: |
As of 01/19/2023, Erytech Pharma’s share price is monitored by Cowen, JMP, Kempen and ODDO BHF, with Jefferies no longer covering Erytech Pharma. The last available rating before this date is ODDO BHF on 11/22/2022, with a “Sell” recommendation and a price target of €3.60. The average price targets of the analysts who follow Erytech Pharma’s stock are as follows*:
| Analyst |
Date | Target (in €) | ||||||
| ODDO BHF |
11/22/2022 | 3.60 | ||||||
| Cowen |
12/14/2021 | N.A. | ||||||
| Jefferies |
11/02/2021 | 2.80 | ||||||
| Average |
3.20 | |||||||
| * | Following the publication of the failure of the Phase III trial of TRYbeCA-1 on 10/25/2021, all analysts have revised their forecasts and recommendations downwards. We have therefore not taken into account the recommendations prior to 10/25/2021. |
The method is indicative, as there has been no regular follow-up by research analysts since the clinical studies were stopped.
| 3. | Valuation methods not used |
| (A) | Net book value |
This method has not been adopted as it does not reflect the company’s current and future losses
118
| (B) | Recent share capital increase |
Erytech Pharma carried out a share capital increase on December 14, 2021, with investors specialized in healthcare. It consisted of a subscription of 769,608 shares with share purchase warrants (ABSA), each ABSA being composed of 4 ADS and 3 BSA, at a price of €2.26 per share.
This method has not been used insofar as since this reserved share capital increase, the company has put an end to its clinical development programs.
| (C) | Stock market comparables / comparable transactions |
In the absence of positive revenues and operating results for Erytech Pharma, as well as for the main comparables, the analogous methods of stock market comparables and comparable transactions could not be applied.
| I. | Valuation of the company Pherecydes Pharma |
| 1. | Principal valuation methods used |
| (A) | Share price analysis |
| Share price |
At 01/19/2023 (in € per share) | |||
| Spot |
2.29 | |||
| Average 20 days |
2.15 | |||
| Average 60 days |
2.21 | |||
| Average 120 days |
2.49 | |||
| Average 250 days |
3.51 | |||
| Max. course 250 days (15/02/2022) |
6.85 | |||
| Course min. 250 days (12/12/2022) |
1.91 | |||
The analysis of Pherecydes Pharma’s share price is performed as of January 19, 2023, which corresponds to the date of signature of the LOI on the Merger. The average of the volume-weighted prices of Pherecydes Pharma shares over the last 60 days preceding the signature of the LOI on the Merger (on January 19, 2023) is equal to €2.21 per Pherecydes Pharma share out of a total of 7,221,477 shares of the company. This average results in a valuation of the company of approximately 16.00 M€.
119
For information, on the eve of the signature of the agreement, the analysis of the liquidity of the Pherecydes Pharma share over the last 24 months is as follows:
| Analysis of the liquidity of Pherecydes Pharma shares as of 05/12/2023 |
||||||||||||||||
| Exchanged securities (as a daily average) |
Average volumes % total |
Exchanged securities (cumulative) |
Cumulative volumes % total |
|||||||||||||
| Spot |
30,370 | 0.42 | % | 30,370 | 0.42 | % | ||||||||||
| 20 days |
5,566 | 0.08 | % | 111,320 | 1.54 | % | ||||||||||
| 60 days |
6,661 | 0.09 | % | 399,650 | 5.53 | % | ||||||||||
| 120 days |
7,167 | 0.10 | % | 860,040 | 11.91 | % | ||||||||||
| 250 days |
6,016 | 0.08 | % | 1,504,080 | 20.83 | % | ||||||||||
| 500 days |
4,537 | 0.06 | % | 2,268,280 | 31.41 | % | ||||||||||
| * | Ratio between the number of shares exchanged and the total number of shares issued at that date (i.e. 7,221,477 shares) . |
Pherecydes Pharma’s share price over the last 500 trading days, as well as the volume of shares traded over this period, are shown in the graph below, for illustrative purposes:

| (B) | Discounted free cash flow (DCF) |
Methodology:
The DCF analysis is based on Pherecydes Pharma’s management projections for the period 2023 to 2042, based on the continued development of its phage therapy indications and a significant financing need to develop these indications in France and internationally.
| • | Only the most advanced indications (Phase I/II or more) and the most likely to be developed in the short and medium term, and for which the clinical study is being conducted by the company itself, were retained for the valuation work. |
| • | The two indications retained are those for phages against Staphylococcus aureus in the treatment of infections of osteoarticular prostheses and the treatment of infective endocarditis. |
120
| • | It has been decided to develop these indications only in Europe, due to the absence of a presence in the US market to date and the difficulty of entering it without a local presence. |
Main Hypothesis:
| • | The valuation date is 01/01/2023. |
| • | Number of shares: 7,323,370 based on the total number of shares issued (excluding treasury shares) and to be issued (in the money at that date). |
| • | The weighted average cost of capital is 17.5%, within the central range of the Biotechnology sector (15%-20%). |
| • | The value of the shares was €20.6 million on January 1, 2023, i.e: |
| • | 8.2 M€ of discounted free cash flows ; |
| • | 12.4 M€ in terminal cash flow to infinity; and |
| • | 1.7 M€ of net debt at 12/31/2022 (gross financial debt adjusted for cash at 12/31/2022). |
On this basis, the free cash flow method gives a center value per share of €2.62.
| 2. | Valuation methods used for information purposes |
| (A) | Share price analysis |
| Share price |
As of 02/15/2023 (in € per share) | |||
| Spot |
2.63 | |||
| Average 20 days |
2.37 | |||
| Average 60 days |
2.26 | |||
| Average 120 days |
2.39 | |||
| Average 250 days |
3.24 | |||
| Max. share price 250 days (15/02/2022) |
5.52 | |||
| Min. share price 250 days (12/12/2022) |
1.91 | |||
For information purposes, the average volume-weighted share price of Pherecydes Pharma over the last 60 days preceding the signature of the MoU on February 15, 2023 is equal to €2.22.
| (B) | Analysts’ price targets |
Portzamparc is the only research analyst to follow the stock since its IPO in early 2021. Portzamparc initiated Pherecydes Pharma’s stock on 04/22/2021 with a price target of €11.10 and gradually lowered it to €7.40 in October 2022.
121
| 3. | Valuation methods not used |
| (A) | Net book value |
This method has not been adopted as it does not reflect the company’s current and future losses.
| (B) | Recent share capital increase |
Pherecydes Pharma carried out a share capital increase on September 22, 2022, with institutional and retail investors. The issue consisted of 1,320,830 new shares with the removal of preferential subscription rights, at a price of €2.32 per share.
The comparison of the share capital increases of Pherecydes Pharma and Erytech Pharma was not relevant insofar as Erytech Pharma reduced its activities to pre-clinical developments after its share capital increase. The method of recent capital transactions was therefore not used.
| (C) | Stock market comparables / comparable transactions |
In the absence of positive revenues and operating income for Pherecydes Pharma, as well as for the main comparables, the analogous methods of stock market comparables and comparable transactions could not be applied.
122
| II. | Synthesis |
The table below summarizes the valuation methods used for Erytech Pharma and Pherecydes Pharma and the resulting parity from the implementation of these methods..
The table also shows the premiums/discounts that the parity chosen by the parties brings out in relation to the parities used in the implementation of the above-mentioned methods:
| Methods |
Erytech Pharma (in €) |
Peherecydes Pharma (in €) |
Induced parity |
Premium/discount externalized in relation to the parity retained by the parties |
||||||||||||
| Primary methods used |
||||||||||||||||
| Share price analysis |
||||||||||||||||
| Spot price (at the signature of the LOI on 01/19/23) |
0.61 | 2.29 | 3.75 | +0.1 | % | |||||||||||
| VWAP 20 days |
0.49 | 2.15 | 4.39 | +17.0 | % | |||||||||||
| VWAP 60 days |
0.51 | 2.21 | 4.33 | +15.6 | % | |||||||||||
| VWAP 120 days |
0.61 | 2.49 | 4.08 | +8.9 | % | |||||||||||
| VWAP 250 days |
1.01 | 3.51 | 3.48 | -7.3 | % | |||||||||||
| Highest price in 250 days |
1.83 | 6.85 | 3.74 | -0.2 | % | |||||||||||
| 250-day lowest price |
0.34 | 1.91 | 5.62 | +49.8 | % | |||||||||||
| Discounted cash flow (DCF) |
||||||||||||||||
| Center value |
0.52 | 2.62 | 5.04 | +34.4 | % | |||||||||||
| Methods used for information purposes |
||||||||||||||||
| Share price analysis |
||||||||||||||||
| Spot price (day of signature of the MoU on 02/15/23) |
0.88 | 2.63 | 2.99 | -20.3 | % | |||||||||||
| VWAP 20 days |
0.92 | 2.37 | 2.58 | -31.3 | % | |||||||||||
| VWAP 60 days |
0.74 | 2.26 | 3.05 | -18.6 | % | |||||||||||
| VWAP 120 days |
0.74 | 2.39 | 3.23 | -13.9 | % | |||||||||||
| VWAP 250 days |
0.97 | 3.24 | 3.34 | -10.9 | % | |||||||||||
| Highest price in 250 days |
1.74 | 5.52 | 3.17 | -15.4 | % | |||||||||||
| 250-day lowest price |
0.34 | 1.91 | 5.62 | +49.8 | % | |||||||||||
| Analysts’ price targets |
||||||||||||||||
| Average price targets (between 10/25/2021 and 01/19/2023) |
3.20 | 7.40 | 2.31 | -38.3 | % | |||||||||||
123
ANNEX 2. REPORTS OF THE MERGER AUDITOR
124

ERYTECH PHARMA
Limited company (société anonyme) with a Board of Directors and capital of
€3,101,855.30
60, avenue Rockefeller
69008, Lyon, France
Lyon Trade and Companies Register no. 479 560 013
PHÉRÉCYDES PHARMA
Limited company (société anonyme) with a Board of Directors and capital of
€7,939,179
22, boulevard Benoni Goullin
44200, Nantes, France
Nantes Trade and Companies Register no. 493 252 266
Merger by absorption of PHÉRÉCYDES PHARMA
by ERYTECH PHARMA
Merger Auditor’s report
on the remuneration of contributions
Order of the Presiding Judge
of the Commercial Court of Lyon
of February 28, 2023


Merger Auditor’s report on the remuneration of the contributions to be
made by PHÉRÉCYDES PHARMA to ERYTECH PHARMA
Dear Sir or Madam,
In accordance with the assignment entrusted to us by the Presiding Judge of the Commercial Court of Lyon on February 28, 2023, with regard to the merger by absorption of PHÉRÉCYDES PHARMA by ERYTECH PHARMA, we have prepared this report on the assessment of the exchange ratio used to determine the remuneration of the contributions, as provided for by Article L.236-10 of the French Commercial Code, it being specified that our assessment of the value of the contributions is the subject of a separate report.
The exchange ratio was set out in the draft merger agreement signed by the representatives of the companies concerned on May 15, 2023 (hereinafter, the “Merger Agreement”).
It is our responsibility to express an opinion on the fairness of the exchange ratio. To this effect, we carried out our procedures according to the professional standards issued by the French Institute of Statutory Auditors (Compagnie nationale des commissaires aux comptes) applicable to this assignment. These professional standards require the implementation of procedures intended both to verify that the relative values assigned to the shares of the companies taking part in the operation are relevant and to analyze the positioning of the exchange ratio compared with the relative values deemed relevant.
As our assignment will end before this report is delivered, it is not our responsibility to update it in order to take into account events and circumstances occurring after its date of signature.
We specify that this report, prepared within the framework of French regulations, does not confer any right under any other law or regulation, even if the Merger is open to shareholders residing in other countries and in particular in the United States.
At no time did we find ourselves in any of the situations of incompatibility, prohibition or forfeiture provided for by law.
2

Please find our observations and conclusions below, presented in the following order:
| 1. | Presentation of the operation |
| 2. | Verification of the relevance of the relative values assigned to the shares of the companies taking part in the operation |
| 3. | Assessment of the fairness of the proposed exchange ratio |
| 4. | Summary – Key points |
| 5. | Conclusion |
3

| 1 | Overview of the proposed operation and description of the contribution |
| 1.1 | Context of the operation |
ERYTECH PHARMA (hereinafter, “ERYTECH” or the “Acquiring Company”) is a clinical-stage biotechnology company developing innovative therapies based on internal research and development programs, to treat patients with diseases in therapeutic areas whose needs are currently not being met, mainly based on red blood cells.
PHÉRÉCYDES PHARMA (hereinafter, “PHÉRÉCYDES” or the “Acquired Company”) is a clinical-stage biotechnology company developing innovative treatments based on internal research and development programs, to treat patients with diseases in therapeutic areas whose needs are currently not being met, mainly based on bacteriophages.
On February 15, 2023, ERYTECH and PHÉRÉCYDES signed a memorandum of understanding (hereinafter, the “MoU”) for the planned merger by absorption of PHÉRÉCYDES by ERYTECH (hereinafter, “the Merger”).
On the same date, certain PHÉRÉCYDES shareholders undertook to contribute, prior to the completion of the Merger and in accordance with the support commitments entered into when the MoU was signed, a portion of their PHÉRÉCYDES shares to the Acquiring Company, in exchange for newly issued ERYTECH shares, according to the same exchange ratio as for the Merger.
The Merger is part of a strategic combination with the aim of creating a global leader in phagotherapy. It aims to leverage the financial resources and teams of PHÉRÉCYDES and ERYTECH to simultaneously accelerate and expand PHÉRÉCYDES’ existing phagotherapy development programs, launch new candidate phages and potentially broaden the scope of application of new therapeutic modalities by using advanced technological platforms and the expertise of both companies.
The financial visibility of the merged entity would last until the third quarter of 2024, with a consolidated cash position of around €41 million as at December 31, 2022, and would enable the financing of multiple clinical stages of some of its existing and future programs.
When the Merger is complete, the former PHÉRÉCYDES shareholders will hold around 49.0% of the merged entity.
4

| 1.2 | Presentation of the companies involved in the operation |
1.2.1. ERYTECH, the Acquiring Company
ERYTECH is a limited company (société anonyme) whose registered office is located at 60, avenue Rockefeller, Lyon (69008), France. It has been entered in the Lyon Trade and Companies Register since November 22, 2004 under number 479 560 013.
Its share capital currently amounts to €3,101,855.30, divided into 31,018,553 shares, each with a nominal value of €0.10, fully paid up and all of the same class.
ERYTECH PHARMA’s shares are admitted to trading on the Euronext Paris market under ISIN code FR0011471135 and on the Nasdaq Securities Market LLC in the form of American Depositary Shares (ADS).
According to its articles of association, ERYTECH has the corporate purpose, “in France and in any country, of:
| • | the research, manufacture, import, distribution, and marketing of experimental drugs, drugs, devices, and medical equipment; |
| • | the provision of all advisory services associated therewith; |
| • | and generally, all financial, commercial, industrial, civil, property, or security-related transactions, such as may directly or indirectly relate to one of the purposes specified or such as may facilitate their fulfillment. |
| • | The Company may act directly or indirectly and perform all these operations in any country, on its own behalf and on behalf of third parties, either alone or with third parties in a joint venture, association, grouping, or company, through the creation of new companies, contributions, partnerships, subscription, purchase of company securities or rights, merger, alliance, joint venture companies, or the obtaining or provision, under lease or management, of any assets and rights or other items.” |
The financial year begins on January 1 and ends on December 31 of each year.
| 1.2.2. | PHÉRÉCYDES, the Acquired Company |
PHÉRÉCYDES is a limited company with its registered office at 22, boulevard Benoni Goullin in Nantes, France (44200). It has been entered in the Nantes Trade and Companies Register since March 23, 2010, under number 493 252 266.
Its share capital currently amounts to €7,939,179, divided into 7,939,179 shares, each with a nominal value of €1.00, fully paid up and all of the same class.
The shares of PHÉRÉCYDES are admitted to trading on the Euronext Growth multilateral trading facility, under ISIN code FR0011651694.
5

According to its articles of association, PHÉRÉCYDES has the corporate purpose of: “in France and abroad:
| • | Developing know-how and obtaining patents and licenses in the field of biology and medicine; |
| • | and more generally in the field of life sciences, on its own behalf or on behalf of third parties, with a view to marketing and distributing its products, as well as any service activity related to the field of life sciences; |
| • | And, generally, all financial, commercial, industrial, securities and real estate transactions that may be directly or indirectly related to the above purpose or to any similar or related purposes, likely to promote its extension or development, including any agreement of any kind with manufacturers or investors.” |
The financial year begins on January 1 and ends on December 31 of each year.
| 1.2.3. | Capital ties between the Acquired Company and the Acquiring Company |
As at the date of the Merger Agreement, no capital ties exist between the Acquiring Company and the Acquired Company.
However, it should be noted that, prior to the date of signature of the Merger Agreement, certain shareholders of the Acquired Company and the Acquiring Company entered into a contribution agreement, under which the aforementioned shareholders of the Acquired Company undertook to contribute 827,132 ordinary shares, i.e. around 10.42% of the capital of PHÉRÉCYDES, to ERYTECH, as set forth in Article 10.1 of the Merger Agreement. In light of this, as the Merger Auditor, we have drawn up two reports, one on the value and the other on the remuneration of the contributions in kind. As at the date of the Merger Agreement, this prior contribution has not yet taken place.
| 1.3 | General terms and conditions of the operation |
The terms and conditions of the Merger, which are described in detail in the Merger Agreement signed by the parties on May 15, 2023 (please see this document for more information), can be summarized as follows.
6

| 1.3.1 | Key characteristics of the Merger |
Completion date and effective date for accounting and tax purposes
The final completion date of the Merger and of the capital increase of the Acquiring Company will be the date of fulfillment of the last of the conditions precedent set out in Article 16 of the Merger Agreement (hereinafter, the “Completion Date”).
The Acquiring Company will be the owner of the asset and liability items contributed by the Acquired Company, including any omitted either in the draft Merger Agreement or in the accounts of the Acquired Company, as of the Completion Date, and the assets of the Acquired Company will be transferred to the Acquiring Company in their present condition on that date.
Completion of the Merger will entail the dissolution without liquidation of the Acquired Company and the universal transfer of its assets to the Acquiring Company.
From an accounting and tax standpoint, the Merger will take effect retroactively on January 1, 2023 (hereinafter, the “Effective Date”). Correspondingly, the results of all asset and liability transactions carried out by the Acquired Company between January 1, 2023 and the Completion Date will exclusively benefit or be charged to the Acquiring Company and considered completed by the Acquiring Company.
Legal jurisdiction
As it is a merger between limited companies (société anonyme), the Merger will take place in accordance with the provisions of Articles L.236-1 to L.236-6 and L.236-8 to L.236-15 of the French Commercial Code.
Tax regime
As regards corporate tax, the parties represent that the Merger will be subject to the preferential tax regime provided for by Article 210 A of the French General Tax Code. Consequently, the unrealized capital gains on all of the fixed assets contributed, as well as the provisions (other than those that become irrelevant), will not be subject to corporate tax at the level of the Acquired Company pursuant to the provisions of Article 210 A of the French General Tax Code.
As regards registration fees, the parties represent that the Merger is subject to the regime provided for in Articles 635, paragraphs 1-5 and 816 of the French General Tax Code. Consequently, the Merger will be registered free of charge within a period of one month from the Completion Date.
Where applicable, the transfer of any real estate deed shall be subject, upon its registration, to a real estate security contribution of 0.1% of the market value of said real estate, in accordance with Articles 879 and 881 K of the same Code.
7

| 1.3.2 | Conditions precedent |
The completion of the Merger and the resulting capital increase of the Acquiring Company are subject to fulfillment of the conditions precedent mentioned in Article 16 of the Merger Agreement, concerning:
| • | the delivery by the Merger Auditor of (i) a report on the value of the contributions and (ii) a report on the terms and conditions of the Merger, confirming the fairness of the exchange ratio adopted; |
| • | the approval by the General Meeting of Shareholders of the Acquired Company of the Merger and the consequent dissolution of the Acquired Company; |
| • | the approval by the General Meeting of Shareholders of the Acquiring Company of the Merger and the capital increase allowing for remuneration of the Merger; and |
| • | the approval by the General Meeting of Shareholders of the Acquiring Company of resolutions on the appointment of directors designated by PHÉRÉCYDES and the amendment of the articles of association of ERYTECH relating to the elimination of the casting vote of the Chairman of the Board of Directors. |
Should the conditions precedent not be fulfilled by midnight on July 31, 2023 at the latest, the Merger Agreement will be automatically considered null and void, with no compensation on either side, unless these conditions are waived by both parties.
8

| 1.4 | Description and valuation of the contributions |
| 1.4.1 | Valuation method |
As this is a regular merger involving entities under separate control, pursuant to French Accounting Standards Authority (Autorité des normes comptables – ANC) regulation no. 2014-03 of June 5, 2014 on the general chart of accounts, as last amended by ANC regulation no. 2022-01 of March 11, 2022, the parties have agreed to retain the actual value of the assets contributed by the Acquired Company and of the liabilities assumed by the Acquiring Company as part of the Merger, as the contribution value.
The contributions will comprise all of the items constituting the assets and liabilities of PHÉRÉCYDES as at the Completion Date.
Details of the valuation methods used to determine the actual value of the asset and liability items are provided in Annex 14.1 of the Merger Agreement and summarized in section 2.2 below.
The net asset value transferred is set at €16,537,386.
| 1.4.2 | Remuneration of contributions |
The remuneration of the contributions corresponding to the net assets of PHÉRÉCYDES is based on the exchange ratio adopted by the parties, determined by reference to the relative values of the PHÉRÉCYDES shares, on the one hand, and of the ERYTECH shares, on the other.
The relative values of the PHÉRÉCYDES shares contributed and the ERYTECH shares issued as remuneration for the contribution are the result of free negotiation between independent parties, supported by the application of a multiple-criteria valuation approach.
Following their negotiation, the relative values of the PHÉRÉCYDES and ERYTECH shares were set by the parties at €2.29 and €0.61 per share, respectively, with reference the market spot prices of the two companies as of January 19, 2023, the date of signature of the Letter of Intent (“LOI”), and to a multi-criteria valuation approach of the two companies.
On this basis, the parties adopted, within the context of the Merger, an exchange ratio of 4 PHÉRÉCYDES shares for 15 ERYTECH shares.
9

Pursuant to the provisions of Article L.236-3 II of the French Commercial Code, there will be no exchange of the PHÉRÉCYDES shares held by the Acquiring Company after the contributions in kind made by certain PHÉRÉCYDES shareholders prior to the completion of the Merger or any exchange of the treasury shares held by PHÉRÉCYDES. These shares will be automatically canceled on the Completion Date.
The Acquiring Company will therefore only increase its capital in order to remunerate the shareholders of the Acquired Company for the fraction of net assets corresponding to the shares that it does not hold in the Acquired Company on the Completion Date.
On the basis of:
| • | a number of PHÉRÉCYDES shares of 7,939,179 comprising the share capital at the date of this report; |
| • | a number of treasury shares of 25,142 on the same date; |
| • | the 827,132 PHÉRÉCYDES shares held by ERYTECH following the contribution in kind; |
| • | the exchange ratio; |
consideration will be made for the contributions via the allocation, to the shareholders of the Acquired Company, of 26,575,893 new shares with a nominal value of €0.10 each, to be issued by the Acquiring Company, which will increase its capital by €2,657,589.30, combined with a balancing payment of €0.42.
The difference between the amount of the portion of remunerated net assets, i.e. €14,757,430.84, and the amount of the capital increase of the Acquiring Company, with a balancing payment of €0.42, i.e. €2,657,589.72, will constitute a merger premium of €12,099,841.12, which will be accounted for as a liability of the Acquiring Company.
It should be noted that the final number of new shares to be issued, and, correspondingly, the nominal amount of the resulting capital increase, will be automatically adjusted according to the exact number of PHÉRÉCYDES shares to be remunerated as part of the Merger, particularly if their amount is adjusted due to any operation that takes place between the date of the Merger Agreement and the Completion Date.
The newly issued ERYTECH shares will carry dividend rights as of the Completion Date, will be exactly the same as the existing ERYTECH shares and will confer the same rights and incur the same charges, including all tax withholdings, so that all ERYTECH shares without exception will confer the right to payment of the same net sum during any distributions, allocations or redemptions during the term of the Acquiring Company or during its liquidation.
10

| 2 | Verification of the relevance of the relative values assigned to the shares of the companies taking part in the operation |
| 2.1 | Procedures performed |
Our assignment is one of the other procedures defined by law and provided for in the conceptual framework of the professional standards of the French Institute of Statutory Auditors.
Its purpose is to provide clarification to the shareholders of the Acquiring Company and of the Acquired Company on the relative values used to determine the exchange ratio, and to assess the fairness of this exchange ratio. Consequently, it is not defined as an audit assignment or a limited review assignment. It also does not involve the validation of the tax regime applicable to the operation. It cannot be assimilated to the due diligence carried out for a lender or an acquirer and does not include all the work necessary for this type of service. Therefore, our report cannot be used in this context.
Likewise, our work is not comparable to that of an independent expert appointed by the administrative or supervisory body of one of the parties.
In carrying out the assignment entrusted to us, we performed the procedures that we considered necessary with regard to the professional standards of the French Institute of Statutory Auditors in order to assess the relevance of the relative values assigned to the shares of the Acquired Company and to the shares of the Acquiring Company and the fairness of the exchange ratio adopted on the basis of these relative values.
In this context, we performed the following procedures in particular:
| • | We interviewed representatives of ERYTECH and PHÉRÉCYDES and their advisors, both to gain knowledge of the proposed operation and its context and to analyze the accounting, financial and legal arrangements envisaged; |
| • | We examined the Merger Agreement and its appendices on May 15, 2023; |
| • | We examined the legal and financial documentation connected with the operation, including the memorandum of understanding (MoU) and its appendices and the Draft Exemption Document to be sent to the French Financial Markets Authority (Autorité des marchés financiers – AMF); |
| • | We reviewed the legal documentation relating to PHÉRÉCYDES and the Acquiring Company; |
| • | We carefully read the consolidated financial statements of ERYTECH as at December 31, 2022, and examined the statutory auditors’ report prepared as part of their audit of the consolidated financial statements for this financial year, making sure that this report did not contain any reservations; |
11

| • | We carefully read the corporate financial statements of PHÉRÉCYDES as at December 31, 2022, and examined the statutory auditor’s report prepared as part of its audit of the annual financial statements, making sure that this report did not contain any reservations; |
| • | We reviewed the budget and forecast data prepared by the management teams of PHÉRÉCYDES and ERYTECH and interviewed the managers concerned to discuss the relevance of the assumptions adopted; |
| • | We examined Appendix 14.1 of the Merger Agreement, which defines, inter alia, the approaches used to determine the relative values of the shares of the Acquiring Company and of the Acquired Company; |
| • | We analyzed and reviewed with the financial consultancy mandated for the Merger (ODDO BHF) the valuation elements for PHÉRÉCYDES and ERYTECH, which appear in Appendix 14.1 of the Merger Agreement and in its valuation report supporting these analyses; |
| • | We analyzed the relevance of the valuation approaches adopted by the parties and the parameters used, then implemented similar or alternative valuation methods and performed sensitivity tests on the exchange ratio used for the remuneration of the contribution for each of the valuation approaches, according to criteria deemed relevant. |
We obtained a letter of representation for PHÉRÉCYDES and ERYTECH, mainly relating to the material elements used in our assignment.
| 2.2 | Relative values used by the parties |
The relative values of the PHÉRÉCYDES shares and the ERYTECH shares that serve to determine the exchange ratio as part of the Merger are the result of free negotiation between independent parties, supported by the implementation of a multiple-criteria approach to the valuation of the shares of the companies involved.
| 2.2.1 | Valuation of the shares of the Acquired Company |
At the end of their negotiations, the relative value of the PHÉRÉCYDES shares was set by the parties at €2.29, by reference to the market spot price of PHÉRÉCYDES on January 19, 2023, i.e. the date of signature of the Letter of Intent (“LOI”), and to a multiple-criteria valuation approach.
12

The parties carried out a multiple-criteria valuation of PHÉRÉCYDES based on:
| • | Reference to the stock price; |
| • | An intrinsic approach based on discounted cash flows |
| • | Reference to the values expressed by the analysts who monitor the PHÉRÉCYDES share. |
The parties excluded:
| • | Reference to the net assets as at December 31, 2022; |
| • | Analogous approaches based on stock market comparables and comparable transactions; |
| • | Recent capital increases. |
The PHÉRÉCYDES values per share obtained using the valuation methods implemented as part of the multiple-criteria approach form the basis of the relative value adopted by the parties.
| Approach |
Value per share (in € per share) |
|||||||
| Min. | Max. | |||||||
| Market spot price as of 01/19/2023 |
2.29 | |||||||
| Principal valuation methods: |
||||||||
| Discounted cash flows |
2.41 | 2.84 | ||||||
| Stock price – VWAP (01/19/2023) |
2.15 | 3.51 | ||||||
| Valuation method for information purposes: |
||||||||
| Stock price – VWAP (02/15/2023) |
2.22 | 3.26 | ||||||
| Analysts’ price targets |
7.40 | 7.40 | ||||||
| 2.2.2 | Valuation of the shares of the Acquiring Company |
To set the number of ERYTECH to be issued as remuneration for the Merger, the parties adopted, at the end of their negotiation, a value per share for this company of €0.61, by reference to the Erytech market spot price on January 19, 2023 and to a multiple-criteria valuation approach.
13

The parties carried out a multiple-criteria valuation of ERYTECH based on:
| • | Reference to the stock price; |
| • | An intrinsic approach based on discounted cash flows |
| • | Reference to the externalized values of analysts monitoring ERYTECH shares. |
The parties excluded:
| • | Reference to the net assets as at December 31, 2022; |
| • | Analogous approaches based on stock market comparables and comparable transactions; |
| • | Recent capital increases. |
The ERYTECH values per share obtained using other valuation methods implemented as part of the multiple-criteria approach form the basis of the relative value adopted by the parties.
| Approach |
Value per share (in € per share) |
|||||||
| Min. | Max. | |||||||
| Market spot price as of 01/19/2023 |
0.61 | |||||||
| Principal valuation methods: |
||||||||
| Discounted cash flows |
0.52 | 0.52 | ||||||
| Stock price – VWAP (01/19/2023) |
0.49 | 1.01 | ||||||
| Valuation method for information purposes: |
||||||||
| Stock price – VWAP (02/15/2023) |
0.74 | 0.97 | ||||||
| Analysts’ price targets |
3.20 | 3.20 | ||||||
| 2.3 | Assessment of the relevance of the relative values |
We have the following observations to make on the assessment of the relative values assigned by the parties:
| • | The relative values adopted by the parties are based on the market spot prices of the two companies on January 19, 2023, the date of signature of the Letter of Intent and the determination of the Merger parity, and on a multiple-criteria valuation approach applied to the two companies; |
14

| • | The DCF approach taken by the parties as the main approach and the reference to the stock price on February 15, 2023, as well as the reference to analysts’ price targets as an indicative approach, form the basis of the relative values adopted. |
| • | We examined the reasons for the exclusion of certain valuation methods and we believe that these criteria were correctly excluded. |
As part of our assignment, we implemented alternative valuation approaches or approaches similar to those adopted by the parties based on our own valuation parameters and carried out sensitivity analyses.
In addition, we carried out a multiple-criteria valuation of PHÉRÉCYDES and ERYTECH based on the following approaches:
Main approach:
| • | An approach based on the intrinsic values of PHÉRÉCYDES and ERYTECH. |
For PHÉRÉCYDES, we used a DCF approach based on management projections, which take into account, in particular (i) the capacity of PHÉRÉCYDES to finance its development on a standalone basis in view of the cash consumption expected at the start of the period, and (ii) the probability of the successful development and marketing of the various treatments.
As regards ERYTECH, the company has no projects in progress and is therefore not expected to generate any revenues in the next few years on a standalone basis (i.e. without taking into account the envisaged merger with PHÉRÉCYDES). Under these conditions, we favored a liquidation-based approach to the value of ERYTECH, considering that on a standalone basis, the value that would accrue to shareholders would correspond to the cash (liquidation bonus) that could be distributed to them after the sale of the company’s assets, collection of receivables and payment of all liabilities and costs relating to the liquidation.
This theoretical approach makes it possible in this context to maximize the value of the company for the shareholders, compared with a DCF approach, which does not appear relevant in a standalone situation, in the absence of new projects, as it would result in a financial imbalance due to an absence of turnover and ongoing high structural costs.
| • | An approach based on the stock prices of PHÉRÉCYDES and ERYTECH on January 19, 2023 (1-month, 60-day, 6-month and 12-month spot prices and VWAPs). |
Secondary approach:
| • | An approach based on the stock prices of PHÉRÉCYDES and ERYTECH on February 15, 2023 (1-month, 60-day, 6-month and 12-month spot prices and VWAPs), the date on which the operation was announced to the market (after close of trading). |
15

The analysis of the stock price on February 15, 2023 is presented on a secondary basis due to the substantial increase in the ERYTECH stock price between late January and early February 2023 (€0.51 at the close of trading on January 25, 2023 and €1.14 at close of trading on February 3, 2023), with higher than average trading volumes. This sudden and unexplained change in the stock price without any announcement on the company’s activity or any specific event affecting it appears likely to limit the relevance of the analysis at February 15, 2023.
It should also be mentioned that, between the date on which the operation was announced (February 15, 2023) and the date of this report, the ERYTECH stock price was very volatile, with trading volumes in certain periods much higher than those historically observed, including 6.9 million shares traded in a three-day period in May 2023. This level of volatility was not observed in the PHÉRÉCYDES stock price. ERYTECH’s stock price is therefore, from our point of view, speculative and does not seem to be a relevant reference point after the announcement of the operation.
Indicative approach:
| • | An approach based on the latest target prices published by analysts before February 15, 2023 (the date on which the operation was announced to the market, after close of trading), it being specified that the companies involved are subject to limited and irregular monitoring, which is why this approach is presented on an indicative basis only. |
| 2.3.1 | Excluded methods |
Our work led us to exclude the same methods as those not adopted by the parties, i.e.:
| 2.3.2 | Net asset value |
Net asset value is not generally considered representative of the intrinsic value of a company, as it does not include growth outlook and profitability, or any capital gains on asset items. Accordingly, this valuation criterion was not adopted in our analyses.
For information purposes, the net asset value of PHÉRÉCYDES as at December 31, 2022 was €7,522,157, i.e. a value per share of €1.04 (on the basis of the number of shares comprising the share capital on that date).
The consolidated net asset value of ERYTECH as at December 31, 2022 was €23,487,000, i.e. a value per share of €0.76 (on the basis of the number of shares comprising the share capital on that date). It should be noted that ERYTECH announced on May 9, 2023 that its cash position as at March 31, 2023 amounted to €30.5 million, representing a decrease of €8.3 million compared with the figure as at December 31, 2022, on the basis of which our work was performed. Therefore, the value per share based on the net asset value as at December 31, 2022, adjusted for this decrease in cash in the first quarter of 2023, would be approximately €0.52 per share.
16

| 2.3.3 | Revalued net asset value |
The revalued net asset value method consists of correcting the net asset value for unrealized capital gains or losses identified in assets, liabilities or off the balance sheet. This method, often used to value companies in certain sectors (holding companies, real estate companies), is particularly suitable for companies whose main assets have a value on a market regardless of whether they are part of an operating process, which is not the case for PHÉRÉCYDES and ERYTECH.
| 2.3.4 | Analogous approaches based on stock market comparables and comparable transactions |
Similar methods based on stock market comparables and comparable transactions consist of determining the value of a company by applying multiples observed on a sample of other companies listed in the same business segment, or multiples externalized during total or partial takeovers of listed or unlisted companies in the business segment of the entity being valued.
As PHÉRÉCYDES’ activity is being developed and as ERYTECH currently has no projects in progress, neither of these companies has normal aggregates (turnover, EBITDA or EBIT) that would enable the application of stock market or transaction multiples. These methods are therefore not applicable.
| 2.3.5 | Reference to recent capital increases |
This method consists of valuing a company by referring to recent material transactions on its capital (excluding analysis of the stock price, which constitutes a separate valuation criterion examined elsewhere).
This method was not adopted, as ERYTECH’s last capital increase took place a relatively long time ago (subscription in December 2021 for 769,608 shares with stock warrants (ABSA), with each of shares comprising four ADSs and three share subscription warrants (BSA), at a price of €2.26 per share), and the company has since ended its main clinical development projects. In these circumstances, a comparison with the last capital increase of PHÉRÉCYDES, which took place in September 2022 at a price of €2.32 per share, does not seem relevant.
17

| 2.3.6 | Common parameters in the valuation methods used |
We performed our valuation work on the basis of the financial statements of the two companies for the period ended December 31, 2022 (corporate financial statements for PHÉRÉCYDES and consolidated financial statements for ERYTECH).
The impact of dilutive instruments in any currency that may exist at the boundaries of the two companies was also incorporated into our work.
| 2.3.7 | Intrinsic approach based on discounted cash flows and net asset value (main approach) |
PHÉRÉCYDES
This method consists of determining the intrinsic value of a company by discounting the cash flows resulting from its business plan at a rate which reflects the profitability requirement of the market vis-à-vis the company, taking into account an exit value at the end of this plan.
This method makes it possible to recognize the value attributable to the development outlook of the company being valued and seems to us appropriate for the situation of PHÉRÉCYDES.
Our work was carried out on the basis of management projections for the period 2023–2042, which take into account, in particular (i) the capacity of PHÉRÉCYDES to finance its development on a standalone basis in view of the cash consumption expected at the start of period, and (ii) the probability of the successful development and marketing of the various treatments.
The transition from enterprise value to equity value used in the context of our work is based on the financial statements as at December 31, 2022 and mainly includes financial borrowings and debt items, from which are deducted cash and cash equivalents, as well as various adjustments regarded as potentially affecting cash or financial debt and not reflected in the aforementioned management projections.
The cash flows were discounted at the weighted average cost of capital, reflecting the risk of execution of the projections. It should be noted that these include the probability of the successful development and marketing of treatments undergoing clinical studies. Sensitivity tests were carried out on the discount rate and the long-term growth rate.
ERYTECH
As previously mentioned, this company has no projects in progress and is therefore not expected to generate any revenues in the next few years on a standalone basis (i.e. without taking into account the envisaged merger with PHÉRÉCYDES).
18

In these circumstances, a DCF approach does not appear relevant in the absence of new projects: in fact, this would result in a financial imbalance due to an absence of turnover and high structural costs.
We therefore favored a liquidation approach to the value of ERYTECH, consisting of determining the cash (liquidation bonus) that may be distributed to shareholders after the sale of the company’s assets, collection of receivables and payment of all liabilities and costs relating to the liquidation.
Our work was carried out on the basis of the assets and liabilities recognized as at December 31, 2022, an estimate of the costs to be incurred for the liquidation (in particular for delisting) and an estimate of the proceeds likely to be collected by the company (in particular for the sale of the Adénine building and intellectual property).
According to this approach, the PHÉRÉCYDES share price ranges between €2.13 and €2.46 and the ERYTECH share price is €0.57, which appears favorable in view of the decrease in cash in the first quarter of 20236.
| 2.3.8 | Stock prices of PHÉRÉCYDES and ERYTECH |
The change in the PHÉRÉCYDES stock price and the ERYTECH stock price rebased to 100 over the 24 months preceding the operation (i.e. up to February 15, 2023) is shown below.

In the period from February 15, 2021 to February 15, 2023, a decrease in the share prices of the two companies can be observed of 76% and 94% respectively for PHÉRÉCYDES and ERYTECH.
| 6 | A decrease of €8.3 million, i.e. an impact of approximately €0.24 per share, all other things being equal. |
19

The change in the ERYTECH stock price should be viewed in light of the announcement, on October 25, 2021, of the failure of the phase III clinical study and its consequences for the activity of the company, which has no more projects in development.
We can also see that the PHÉRÉCYDES stock price was relatively stable between October 2022 and the date on which the operation was announced, even though the market had been informed of the short-term cash situation7 and company’s financing requirements.
Analysis of January 19, 2023 (main analysis)
As our main analysis, we examined the change in the respective stock prices of PHÉRÉCYDES and ERYTECH in the 12 months prior to the signing of the letter of intent and the determination of the parity for the Merger (i.e. before January 19, 2023).
During this period, the volume-weighted average prices (VWAPs) of the shares were as follows:
| Valuation method |
Phérécydes share value (€) |
Erytech share value (€) |
||||||
| Spot price at signature of LOI – 01/19/2023 |
2.29 | 0.61 | ||||||
| 1-month VWAP |
2.15 | 0.48 | ||||||
| 60-day VWAP |
2.21 | 0.50 | ||||||
| 6-month VWAP |
2.51 | 0.64 | ||||||
| 1-year VWAP |
3.51 | 1.02 | ||||||
Using this approach, the PHÉRÉCYDES share price ranges between €2.15 and €3.51 and the ERYTECH share price between €0.48 and €1.02.
| 7 | On September 22, 2022, Phérécydes announced, via a press release, a capital increase of €3.1 million, providing it with financing until March 31, 2023, and estimated additional cash requirements of €4 million to finance its operations until September 31, 2023. |
On October 31, 2022, in its half-year financial report, Phérécydes adjusted its projected financing requirements to between €5 million and €6 million in order to continue operating until the end of October 2023.
On February 17, 2023, Phérécydes announced that it had carried out an additional capital increase of €1.5 million, enabling it to continue operating until June 30, 2023, the scheduled completion date for the Merger.
20

Analysis of February 15, 2023 (secondary analysis)
The analysis of changes in the respective stock prices of PHÉRÉCYDES and ERYTECH in the 12 months leading up to the announcement of the operation (i.e. up to February 15, 2023) gives the following VWAPs:
| Valuation method |
Phérécydes share value (€) |
Erytech share value (€) |
||||||
| Spot price at announcement of the operation – 02/15/2023 |
2.63 | 0.875 | ||||||
| 1-month VWAP |
2.37 | 0.89 | ||||||
| 60-day VWAP |
2.24 | 0.74 | ||||||
| 6-month VWAP |
2.39 | 0.74 | ||||||
| 1-year VWAP |
3.25 | 0.97 | ||||||
It should be noted that this is presented as a secondary analysis for the reasons provided above (see section 2.3).
Using this approach, the PHÉRÉCYDES share price ranges between €2.24 and €3.25 and the ERYTECH share price between €0.74 and €0.97.
| 2.3.9 | Analyst target share prices (for information purposes) |
PHÉRÉCYDES and ERYTECH are subject to limited and irregular monitoring by analysts: this criterion is therefore presented for guidance purposes only.
We performed our analysis on the basis of the last share price targets published between October 25, 2021 and February 15, 2023, in order to take into account the adjustments to the ERYTECH share price targets following the announcement on October 25, 2021, of the failure of Erytech’s phase III clinical study and before the operation was announced to the market after close of trading on February 15, 2023.
In this period, only one analyst published a share price target for PHÉRÉCYDES and two analysts published targets for ERYTECH.
| Latest analyst price targets |
Date | Erytech | Phérécydes | |||||||||
| ODDO BHF |
11/22/2022 | 3.60 | € | N/A | ||||||||
| Portzamparc |
10/28/2022 | N/A | 7.40 | € | ||||||||
| Jefferies |
11/02/2021 | 2.80 | € | N/A | ||||||||
|
|
|
|
|
|
|
|||||||
| Average | 3.20 € | 7.40 € | ||||||||||
|
|
|
|
|
|||||||||
According to this approach, the PHÉRÉCYDES share price is €7.40 and the ERYTECH share price is €3.20.
21

In summary, our work does not call into question the relative values adopted by the parties, i.e. €2.29 per PHÉRÉCYDES share and €0.61 per ERYTECH share.
| 3 | Assessment of the fairness of the proposed exchange ratio |
| 3.1 | Proposed remuneration of the contribution |
In a context of negotiation between independent third parties, and on the basis of the multiple-criteria analysis performed, the parties have set an exchange ratio of 4 PHÉRÉCYDES shares for 15 ERYTECH shares (i.e. 1 PHÉRÉCYDES share for 3.75 ERYTECH SHARES).
| 3.2 | Procedures performed |
We performed the procedures that we deemed necessary in order to comply with the professional standards issued by the French Institute of Statutory Auditors to assess the fairness of the proposed exchange ratio. To this end, we have:
| • | Analyzed the positioning of the exchange ratio in relation to the relative values deemed relevant, determined by reference to a multiple-criteria approach. |
In particular, we relied on the work described in section 2.3 above, which we carried out in order to verify the relevance of the relative valuesattributed to the shares of the Acquired Company and of the Acquiring Company.
| • | Understood the impact of the exchange ratio on the future situation of the shareholders of the two companies. |
On this basis, we assessed the fairness of the proposed exchange ratio.
| 3.3 | Assessment and positioning of the proposed exchange ratio |
At the end of their discussions, the parties agreed to set the exchange ratio by directly comparing the relative values derived (i) from the market spot prices of the two companies as at January 19, 2023, and (ii) a multiple-criteria valuation approach applied to the two companies.
22


The exchange ratio agreed between the parties falls within the range of the analysis of the stock price as at January 19, 2023 (spot price and 1-month to 12-month VWAPs) and is based on the intrinsic approach and the analysis of the stock price as at February 15, 2023.
On completion of our work, we have no observations to make on the exchange rate of 4 PHÉRÉCYDES shares for 15 ERYTECH shares (i.e. 1 PHÉRÉCYDES share for 3.75 ERYTECH SHARES) adopted by the parties.
23

| 3.4 | Consequences of the Merger for the shareholders of the Acquiring Company and of the Acquired Company |
The aim of the Merger is to give financial visibility to the new entity, with a consolidated cash position of around €41 million as at December 31, 2022, and to leverage the teams of PHÉRÉCYDES and ERYTECH to simultaneously accelerate and expand the existing phagotherapy development programs of PHÉRÉCYDES, launch new candidate phages and potentially broaden the scope of application of new therapeutic modalities by using advanced technological platforms and the expertise of both companies.
To assess the fairness of the operation, it is useful to examine, in addition to its industrial interest, the operational synergies that it is likely to generate.
The parties believe that the operation should generate cost synergies, the full effect of which is expected from the second year after the completion of the Merger, after having incurred implementation costs in the first year.
These synergies correspond to the savings that may be made by optimizing structural costs and the delisting of PHÉRÉCYDES.
These synergies are not included in our assessment of the relative values of PHÉRÉCYDES and ERYTECH, and will benefit all shareholder groups.
We simulated the impact of these synergies on the value per share on the basis of a combined business plan for the two companies, taking into account the number of shares comprising the capital of ERYTECH after completion of the Merger.
The analysis shows significant potential value creation for the two groups of shareholders compared with the 12-month VWAPs as at January 19, 2023, it being specified that these analyzes are based on the synergy projections and estimates communicated by the management teams of the Acquiring Company and the Acquired Company.
It should be noted that we have not carried out any analysis of accretion or dilution of net earnings per share, as neither of the companies in question generates a net profit.
24

| 4 | Summary – Key points |
The aim of this Merger operation is to enable ERYTECH to contribute to the development of the projects of the merged entity, potentially creating value for its shareholders.
For the PHÉRÉCYDES shareholder, the aim of the operation is to enable the development program to continue with the benefit of the financial and human resources of ERYTECH. It will be recalled that, before the MoU was signed, PHÉRÉCYDES was seeking financing for its clinical development programs, a situation that was known to the market.
According to the press release published jointly by the two companies on February 15, 2023, the financial visibility of the new entity created by the Merger will last until the third quarter of 2024, with a consolidated cash position of around €41 million as at December 31, 2022, enabling the financing of multiple clinical stages of some of its existing and future programs.
The two parties negotiated the financial terms of the Merger independently. The proposed exchange ratio was determined according to a share price for PHÉRÉCYDES of €2.29 and a share price for ERYTECH of €0.61. We have the following observations to make on these financial terms:
| • | The exchange ratio adopted by the parties is based in particular on the market spot prices of the two companies as at January 19, 2023, the date of signature of the Letter of Intent and the determination of the Merger parity; |
| • | The exchange ratio is within the range of the volume-weighted average prices as at January 19, 2023; |
| • | The exchange ratio falls between the valuation range of our valuation approach, based on the discounted future cash flows of PHÉRÉCYDES and the net asset value of ERYTECH, which are, in our opinion, the most relevant approaches; |
| • | The exchange ratio is also at the top end of the range of the analysis of the stock price as at February 15, 2023, which we are presenting on a secondary basis due to the limitations described above. It should also be noted that reference to the stock price after this date of February 15, 2023 does not seem relevant to us, given the volatility observed in the ERYTECH stock price since this date. |
Furthermore, according to the analyses performed, the Merger should enable value creation through the implementation of synergies and the pooling of the merged entity’s development programs and the financial resources and know-how of the two companies, which will benefit the shareholders of both parties.
25

| 5 | Conclusion |
On the basis of our work at as at the date of this report, we are of the opinion that the exchange ratio of 4 PHÉRÉCYDES shares for 15 ERYTECH shares is fair.
Paris, May 15, 2023
The Merger Auditor
FINEXSI EXPERT & CONSEIL FINANCIER
Christophe Lambert
Statutory Auditor
Member of the Paris Regional Association of Statutory Auditors
(Compagnie Régionale de Paris)
26

ERYTECH PHARMA
Limited company (société anonyme) with a Board of Directors and capital of
€3,101,855.30
60, avenue Rockefeller
69008, Lyon, France
Lyon Trade and Companies Register no. 479 560 013
Merger by absorption of PHÉRÉCYDES PHARMA by ERYTECH PHARMA
Merger Auditor’s report
on the value of the contributions
Order of the Presiding Judge
of the Commercial Court of Lyon
of February 28, 2023


Merger Auditor’s report
on the value of the contributions to be made by PHÉRÉCYDES PHARMA to
ERYTECH PHARMA
Dear Sir or Madam,
In accordance with the assignment entrusted to us by the Presiding Judge of the Commercial Court of Lyon on February 28, 2023, with regard to the merger by absorption of PHÉRÉCYDES PHARMA by ERYTECH PHARMA, we hereby report on the value of the contributions provided for by Articles L.236-10 and L.225-147 of the French Commercial Code, it being specified that our assessment of the fairness of the exchange ratio is the subject of a separate report.
The net assets contributed by PHÉRÉCYDES PHARMA to ERYTECH PHARMA were set out in the draft merger agreement signed by the representatives of the companies concerned on May 15, 2023 (hereafter the “Merger Agreement”).
It is our responsibility to reach a conclusion as to whether the value of the contributions is overstated. To this effect, we carried out our procedures according to the professional standards issued by the French Institute of Statutory Auditors (Compagnie nationale des commissaires aux comptes) applicable to this assignment. These professional standards require that due diligence be performed to assess the value of the contributions, to ensure that it is not overvalued and to verify that it corresponds at least to the nominal value of the shares to be issued by the Acquiring Company, plus the merger premium.
As our assignment will end before this report is delivered, it is not our responsibility to update it in order to take into account events and circumstances occurring after its date of signature.
At no time did we find ourselves in any of the situations of incompatibility, prohibition or forfeiture provided for by law.
We specify that this report, prepared within the framework of French regulations, does not confer any right under any other law or regulation, even if the Merger is open to shareholders residing in other countries and in particular in the United States.
2

Please find our observations and conclusions below, presented in the following order:
| 1. | Overview of the proposed operation and description of the contributions |
| 2. | Procedures carried out and assessment of the value of the contributions |
| 3. | Summary – Key points |
| 4. | Conclusion |
3

| 1. | OVERVIEW OF THE PROPOSED OPERATION AND DESCRIPTION OF THE CONTRIBUTIONS |
| 1.1 | Context of the operation |
ERYTECH PHARMA (hereinafter “ERYTECH” or the “Acquiring Company”) is a clinical-stage biotechnology company developing innovative therapies based on internal research and development programs, to treat patients with diseases in therapeutic areas whose needs are currently not being met, mainly based on red blood cells.
PHÉRÉCYDES PHARMA (hereinafter, “PHÉRÉCYDES” or the “Acquired Company”) is a clinical-stage biotechnology company developing innovative treatments based on internal research and development programs, to treat patients with diseases in therapeutic areas whose needs are currently not being met, mainly based on bacteriophages.
On February 15, 2023, ERYTECH and PHÉRÉCYDES signed a memorandum of understanding (hereinafter, the “MoU”) for the planned merger by absorption of PHÉRÉCYDES by ERYTECH (hereinafter, “the Merger”).
On the same date, certain PHÉRÉCYDES shareholders undertook to contribute, prior to the completion of the Merger and in accordance with the support commitments entered into when the MoU was signed, a portion of their PHÉRÉCYDES shares to the Acquiring Company, in exchange for newly issued ERYTECH shares, according to the same exchange ratio as for the Merger.
The Merger is part of a strategic combination with the aim of creating a global leader in phagotherapy. It aims to leverage the financial resources and teams of PHÉRÉCYDES and ERYTECH to simultaneously accelerate and expand PHÉRÉCYDES’ existing phagotherapy development programs, launch new candidate phages and potentially broaden the scope of application of new therapeutic modalities by using advanced technological platforms and the expertise of both companies.
The financial visibility of the merged entity would last until the third quarter of 2024, with a consolidated cash position of around €41 million as at December 31, 2022, and would enable the financing of multiple clinical stages of some of its existing and future programs.
When the Merger is complete, the former PHÉRÉCYDES shareholders will hold around 49.0% of the merged entity.
4

| 1.2 | Presentation of the companies involved in the operation |
| 1.2.1 | ERYTECH, the Acquiring Company |
ERYTECH is a limited company (société anonyme) whose registered office is located at 60, avenue Rockefeller, Lyon (69008), France. It has been entered in the Lyon Trade and Companies Register since November 22, 2004 under number 479 560 013.
Its share capital currently amounts to €3,101,855.30, divided into 31,018,553 shares, each with a nominal value of €0.10, fully paid up and all of the same class.
ERYTECH PHARMA’s shares are listed on the Euronext Paris market under ISIN code FR0011471135 and on the Nasdaq Securities Market LLC in the form of American Depositary Shares.
According to its articles of association, ERYTECH has the corporate purpose, “in France and in any country, of:
| • | the research, manufacture, import, distribution, and marketing of experimental drugs, drugs, devices, and medical equipment; |
| • | the provision of all advisory services associated therewith; |
| • | and generally, all financial, commercial, industrial, civil, property, or security-related transactions, such as may directly or indirectly relate to one of the purposes specified or such as may facilitate their fulfillment. |
| • | The Company may act directly or indirectly and perform all these operations in any country, on its own behalf and on behalf of third parties, either alone or with third parties in a joint venture, association, grouping, or company, through the creation of new companies, contributions, partnerships, subscription, purchase of company securities or rights, merger, alliance, joint venture companies, or the obtaining or provision, under lease or management, of any assets and rights or other items.” » |
The financial year begins on January 1 and ends on December 31 of each year.
| 1.2.2 | PHERECYDES, the Acquired Company |
PHÉRÉCYDES is a limited company with its registered office at 22, boulevard Benoni Goullin in Nantes, France (44200). It has been entered in the Nantes Trade and Companies Register since March 23, 2010, under number 493 252 266.
Its share capital currently amounts to €7,939,179, divided into 7,939,179 shares, each with a nominal value of €1.00, fully paid up and all of the same class.
The shares of PHÉRÉCYDES are admitted to trading on the Euronext Growth multilateral trading facility, under ISIN code FR0011651694.
5

According to its articles of association, PHÉRÉCYDES has the corporate purpose of: “in France and abroad:
| • | Developing know-how and obtaining patents and licenses in the field of biology and medicine; |
| • | and more generally in the field of life sciences, on its own behalf or on behalf of third parties, with a view to marketing and distributing its products, as well as any service activity related to the field of life sciences; |
| • | And, generally, all financial, commercial, industrial, securities and real estate transactions that may be directly or indirectly related to the above purpose or to any similar or related purposes, likely to promote its extension or development, including any agreement of any kind with manufacturers or investors.” » |
The financial year begins on January 1 and ends on December 31 of each year.
| 1.2.3 | Capital ties between the Acquired Company and the Acquiring Company |
As at the date of the Merger Agreement, no capital ties exist between the Acquiring Company and the Acquired Company.
However, it should be noted that, prior to the date of signature of the Merger Agreement, certain shareholders of the Acquired Company and the Acquiring Company entered into a contribution agreement, under which the aforementioned shareholders of the Acquired Company undertook to contribute 827,132 ordinary shares, i.e. around 10.42% of the capital of PHÉRÉCYDES, to ERYTECH, as set forth in Article 10.1 of the Merger Agreement. In light of this, as the Merger Auditor, we have drawn up two reports, one on the value and the other on the remuneration of the contributions in kind. As at the date of the Merger Agreement, this prior contribution has not yet taken place.
6

| 1.3 | General terms and conditions of the operation |
The terms and conditions of the Merger, which are described in detail in the Merger Agreement signed by the parties on May 15, 2023 (please see this document for more information), can be summarized as follows.
| 1.3.1 | Key characteristics of the Merger |
Completion date and effective date for accounting and tax purposes
The final completion date of the Merger and of the capital increase of the Acquiring Company will be the date of fulfillment of the last of the conditions precedent set out in Article 16 of the Merger Agreement (hereinafter, the “Completion Date”).
The Acquiring Company will be the owner of the asset and liability items contributed by the Acquired Company, including any omitted either in the draft Merger Agreement or in the accounts of the Acquired Company, as of the Completion Date, and the assets of the Acquired Company will be transferred to the Acquiring Company in their present condition on that date.
Completion of the Merger will entail the dissolution without liquidation of the Acquired Company and the universal transfer of its assets to the Acquiring Company.
From an accounting and tax standpoint, the Merger will take effect retroactively on January 1, 2023 (hereinafter, the “Effective Date”). Consequently, the results of all operations relating to assets and liabilities, carried out by the Acquired Company as from January 1, 2023 until the Completion Date, will be exclusively for the benefit of or at the expense of the Acquiring Company and will be deemed to have been carried out by the Acquiring Company.
Legal jurisdiction
As it is a merger between limited companies (société anonyme), the Merger will take place in accordance with the provisions of Articles L.236-1 to L.236-6 and L.236-8 to L.236-15 of the French Commercial Code.
Tax regime
As regards corporate tax, the parties represent that the Merger will be subject to the preferential tax regime provided for by Article 210 A of the French General Tax Code. Consequently, the unrealized capital gains on all of the fixed assets contributed, as well as the provisions (other than those that become irrelevant), will not be subject to corporate tax at the level of the Acquired Company pursuant to the provisions of Article 210 A of the French General Tax Code.
7

As regards registration fees, the parties represent that the Merger is subject to the regime provided for in Articles 635, paragraphs 1-5 and 816 of the French General Tax Code. Consequently, the Merger will be registered free of charge within a period of one month from the Completion Date.
Where applicable, the transfer of any real estate deed shall be subject, upon its registration, to a real estate security contribution of 0.1% of the market value of said real estate, in accordance with Articles 879 and 881 K of the same Code.
| 1.3.2 | Conditions precedent |
The completion of the Merger and the resulting capital increase of the Acquiring Company are subject to fulfillment of the conditions precedent mentioned in Article 16 of the Merger Agreement, concerning:
| • | the delivery by the Merger Auditor of (i) a report on the value of the contributions and (ii) a report on the terms and conditions of the Merger, confirming the fairness of the exchange ratio adopted; |
| • | the approval by the General Meeting of Shareholders of the Acquired Company of the Merger and the consequent dissolution of the Acquired Company; |
| • | the approval by the General Meeting of Shareholders of the Acquiring Company of the Merger and the capital increase allowing for remuneration of the Merger; and |
| • | the approval by the General Meeting of Shareholders of the Acquiring Company of resolutions on the appointment of directors designated by PHÉRÉCYDES and the amendment of the articles of association of ERYTECH relating to the elimination of the casting vote of the Chairman of the Board of Directors. |
Should the conditions precedent not be fulfilled by midnight on July 31, 2023 at the latest, the Merger Agreement will be automatically considered null and void, with no compensation on either side, unless these conditions are waived by both parties.
8

| 1.4 | Description and valuation of the contributions |
| 1.4.1 | Valuation method |
As this is a regular merger involving entities under separate control, pursuant to French Accounting Standards Authority (Autorité des normes comptables – ANC) regulation no. 2014-03 of June 5, 2014 on the general chart of accounts, as last amended by ANC regulation no. 2022-01 of March 11, 2022, the parties have agreed to retain the actual value of the assets contributed by the Acquired Company and of the liabilities assumed by the Acquiring Company as part of the Merger, as the contribution value.
The contributions will comprise all of the items constituting the assets and liabilities of PHÉRÉCYDES as at the Completion Date.
Details of the valuation methods used to determine the actual value of the asset and liability items are provided in Annex 14.1 of the Merger Agreement and summarized in section 2.5.1 below.
On this basis, the actual value of the assets contributed by the Acquired Company to the Acquiring Company amounts to €16,537,386, resulting from the designations and valuations of the contributed assets and liabilities presented below.
| 1.4.2 | Description of the assets and liabilities transferred |
In accordance with the provisions of Article L.236-3 of the French Commercial Code, PHÉRÉCYDES will transfer to ERYTECH all of its assets and liabilities in the state in which they are found at the Completion Date of the Merger.
The actual value of the contributed assets and transferred liabilities determined by the parties can be summarized as follows:
Assets contributed:
| Assets |
Actual value (€) | |||
| Intangible assets |
18,087,000 | |||
| Property, plant and equipment |
559,184 | |||
| Financial fixed assets |
149,825 | |||
|
|
|
|||
| Total fixed assets |
18,796,009 | |||
|
|
|
|||
| Inventories |
— | |||
| Receivables |
2,367,790 | |||
| Cash and cash equivalents |
1,035,127 | |||
| Total current assets |
3,435,329 | |||
| Unrealized exchange losses |
— | |||
9

The actual value of the contributed assets is therefore €22,231,338.
Liabilities contributed:
| Liabilities |
Actual value (€) | |||
| Provisions for contingencies and charges |
— | |||
| Financial borrowings and debt |
3,070,146 | |||
| Operating liabilities |
2,383,806 | |||
| Deferred income |
240,000 | |||
| Total financial borrowings and debt |
5,693,952 | |||
| Unrealized exchange profit |
— | |||
The total amount of the liabilities included in the contribution is therefore €5,693,952.
The value of the net assets transferred is therefore €16,537,386.
| 1.4.3 | Remuneration of contributions |
The remuneration of the contributions corresponding to the net assets of PHÉRÉCYDES is based on the exchange ratio adopted by the parties, determined by reference to the relative values of the PHÉRÉCYDES shares, on the one hand, and of the ERYTECH shares, on the other.
The relative values of the PHÉRÉCYDES shares contributed and the ERYTECH shares issued as remuneration for the contribution are the result of free negotiation between independent parties, supported by the application of a multiple-criteria valuation approach.
Following their negotiation, the relative values of the PHÉRÉCYDES and ERYTECH shares were set by the parties at €2.29 and €0.61 per share, respectively, with reference the market spot prices of the two companies as of January 19, 2023, the date of signature of the Letter of Intent (“LOI”), and to a multi-criteria valuation approach of the two companies.
10

On this basis, the parties adopted, within the context of the Merger, an exchange ratio of 4 PHÉRÉCYDES shares for 15 ERYTECH shares. Our assessment of this exchange ratio is the subject of a separate report.
11

Pursuant to the provisions of Article L.236-3 II of the French Commercial Code, there will be no exchange of the PHÉRÉCYDES shares held by the Acquiring Company after the contributions in kind made by certain PHÉRÉCYDES shareholders prior to the completion of the Merger or any exchange of the treasury shares held by PHÉRÉCYDES. These shares will be automatically canceled on the Completion Date.
The Acquiring Company will therefore only increase its capital in order to remunerate the shareholders of the Acquired Company for the fraction of net assets corresponding to the shares that it does not hold in the Acquired Company on the Completion Date.
On the basis of:
| • | a number of PHÉRÉCYDES shares of 7,939,179 comprising the share capital at the date of this report; |
| • | a number of treasury shares of 25,142 on the same date; |
| • | the 827,132 PHÉRÉCYDES shares held by ERYTECH following the contribution in kind; |
| • | the exchange ratio; |
consideration will be made for the contributions via the allocation, to the shareholders of the Acquired Company, of 26,575,893 new shares with a nominal value of €0.10 each, to be issued by the Acquiring Company, which will increase its capital by €2,657,589.30, combined with a balancing payment of €0.42.
The difference between the amount of the portion of remunerated net assets, i.e. €14,757,430.84, and the amount of the capital increase of the Acquiring Company, with a balancing payment of €0.42, i.e. €2,657,589.72, will constitute a merger premium of €12,099,841.12, which will be accounted for as a liability of the Acquiring Company.
It should be noted that the final number of new shares to be issued, and, correspondingly, the nominal amount of the resulting capital increase, will be automatically adjusted according to the exact number of PHÉRÉCYDES shares to be remunerated as part of the Merger, particularly if their amount is adjusted due to any operation that takes place between the date of the Merger Agreement and the Completion Date.
12

The newly issued ERYTECH shares will carry dividend rights as of the Completion Date, will be exactly the same as the existing ERYTECH shares and will confer the same rights and incur the same charges, including all tax withholdings, so that all ERYTECH shares without exception will confer the right to payment of the same net sum during any distributions, allocations or redemptions during the term of the Acquiring Company or during its liquidation.
| 2. | PROCEDURES AND ASSESSMENT OF THE VALUE OF THE CONTRIBUTIONS |
| 2.1 | Procedures carried out by the merger auditors |
Our assignment is one of the other procedures defined by law and provided for in the conceptual framework of the professional standards of the French Institute of Statutory Auditors.
Its purpose is to inform the shareholders of the Acquiring Company that the contributions of the Acquired Company have not been overstated. Consequently, it is not defined as an audit assignment or a limited review assignment. It also does not involve the validation of the tax regime applicable to the operation. It cannot be assimilated to the due diligence carried out for a lender or an acquirer and does not include all the work necessary for this type of service. Therefore, our report cannot be used in this context.
Likewise, our work is not comparable to that of an independent expert appointed by the administrative or supervisory body of one of the parties.
In accordance with the assignment entrusted to us, we have performed the procedures which we considered necessary to comply with the professional guidance issued by the French Institute of Statutory Auditors, in order to verify that the net asset value contributed is not overstated.
To this end, we have:
| • | Ascertained the reality and the ownership of the contributions and assessed the possible impact of elements likely to affect their ownership; |
| • | Assessed the value of the contributions set out in the Merger Agreement; |
| • | Verified compliance with the accounting regulations in force on the valuation of contributions; |
| • | Verified that the actual value of the contributions taken as a whole is at least equal to the overall value of the contributions proposed in the Merger Agreement; |
| • | Verified, up to the date of drafting of this report, the absence of facts or events likely to call into question the overall value of the contributions. |
In particular, the procedures we carried out consisted mainly of:
13

| • | Becoming familiar with the context, objectives and the process that led to the Merger; |
| • | Meeting with the representatives of ERYTECH and PHÉRÉCYDES in order to become familiar with the proposed operation and its context, as well as to analyze the accounting, financial and legal terms and conditions proposed; |
| • | Reviewing the Merger Agreement and its appendices signed by the parties on May 15, 2023; |
| • | Becoming familiar with the legal and financial documentation on the operation, including the memorandum of understanding (MoU) and its appendices and the Draft Exemption Document to be sent to the French Financial Markets Authority (Autorité des marchés financiers – AMF); |
| • | Reviewing the legal documentation related to PHÉRÉCYDES; |
| • | Becoming familiar with the corporate financial statements of PHÉRÉCYDES as at December 31, 2022, examining the statutory auditor’s report drawn up within the framework of its audit of the annual financial statements and ensuring that such report did not contain any reservations; |
| • | Analyzing and reviewing, along with the financial advisor mandated under the Merger (ODDO BHF), the PHÉRÉCYDES valuation elements that appear in Appendix 14.1 of the Merger Agreement, as well as the valuation report underlying these analyses; |
| • | Monitoring the valuation of contributions, reviewing and assessing the valuation methods used, and implementing similar or alternative methods to ensure an appropriate multi-criteria approach that includes the most relevant criteria; |
| • | Reviewing the projections of PHÉRÉCYDES’ management and examining, alongside management, the fundamentals of the business and the growth and operational profitability perspectives with regard to the evolution of the market; |
| • | Obtaining a letter of representation from the representatives of PHÉRÉCYDES and ERYTECH, who have confirmed the material elements used as part of our assignment. |
We have also relied on our work to assess the fairness of the exchange ratio used for the consideration paid for the contributions, which we report on separately.
14

| 2.2 | Assessment of the contribution valuation method and its compliance with accounting regulations |
Under the terms of the Merger Agreement, the parties have agreed to use the actual value of the assets and liabilities making up the net assets of the Acquired Company.
As this is a regular merger involving companies under separate control, it must be carried out at the actual value in accordance with the provisions of ANC regulation no. 2014-03 of June 5, 2014 on the general chart of accounts, as last amended by ANC regulation no. 2022-01 of March 11, 2022.
The choice made in the Merger Agreement regarding the contribution valuation method complies with the aforementioned regulation and does not call for any comments on our part.
| 2.3 | Reality of the contributions |
We have verified that the assets contributed and the liabilities transferred, as identified and described in sections 11.2 and 11.3 of the Merger Agreement and recalled in section 1.4.2 of this report, constituted the assets and liabilities of the Acquired Company as at December 31, 2022, and that the latter was free to use such assets and liabilities as it deemed fit. In addition, we have received confirmation in a letter of affirmation that there are no restrictions on the transfer of such transferred assets and liabilities.
| 2.4 | Assessment of the individual value of the contributions |
The identification of the assets contributed and liabilities assumed has been made on the basis of PHÉRÉCYDES’ annual financial statements as of December 31, 2022.
PHÉRÉCYDES’ annual financial statements as at December 31, 2022 are set out in Appendix 6 of the Merger Agreement and were not the subject of any reservations by the statutory auditor in its report issued on April 26, 2023.
The actual values of the assets and liabilities transferred have been estimated to be equal to their book values according to the balance sheet of the Acquired Company as at December 31, 2022, with the exception of intangible assets, which have been subject to a revaluation of €9,015,228.
The actual value of the intangible assets, i.e. €18,087,000, is made up of €17,070,000 in development costs, determined on the basis of the valuation work carried out by an appraiser commissioned by the Acquiring Company, and €1,017,000 in goodwill, determined by the difference between the actual value of the net assets transferred and the value attributed to the other assets and liabilities
15

As indicated below in our assessment of the overall value of the contributions, we have taken note of the valuation made by the parties in order to determine the actual value of the net assets transferred, i.e. €16,537,386.
We have no comments to make on the individual values of the transferred assets and liabilities.
| 2.5 | Assessment of the overall value of the contributions |
| 2.5.1 | Value used by the parties |
The value of the contributions is the result of a free negotiation between independent parties, which was bolstered by the implementation of a multi-criteria valuation approach.
In this context, ERYTECH’s advisory bank has implemented a multi-criteria valuation approach by focusing on the following methods:
| • | Reference to the stock price; |
| • | Discounted cash flows (DCF); |
| • | Reference to the values expressed by the analysts who monitor the PHÉRÉCYDES share. |
The following methods were not used:
| • | Reference to the net assets as at December 31, 2022; |
| • | Analogous approaches based on stock market comparables and comparable transactions; |
| • | Recent capital increases. |
Below we present the valuation elements detailed in Appendix 14.1 of the Merger Agreement.
16

| (i) | Transition from enterprise value to equity value |
The transition from the enterprise value to the equity value was determined, as at December 31, 2022, on the basis of the net financial debt of PHÉRÉCYDES, adjusted for certain debt-like and cash-like items of PHÉRÉCYDES.
| (i) | Reference to the stock price: |
The stock price is an instrument to measure the price of the company’s shares, freely traded subject to sufficient free float and liquidity levels.
This reference was primarily used as of January 19, 2023, corresponding to the date of signature of the letter of intent (“LOI”) and the determination of the parity as part of the Merger, for the analysis of the stock price. Analyses were also performed on the 20-day, 60-day, 120-day and 250-day VWAP (volume-weighted average price) calculated at the above date.
This reference shows a value per share range of between €2.15 and €3.51, i.e. an overall value of between €15,745,256 and €25,705,029.
For information purposes, the stock price as of February 15, 2023 was also analyzed.
| (ii) | Discounted cash flows (DCF): |
According to this method, the value of an enterprise or business is equal to the sum of (i) the discounted values of the future cash flows that the operation is likely to generate over the business plan horizon and (ii) a terminal value over the horizon of these forecasts determined on the basis of an estimated standard cash flow.
Cash flows are discounted at a rate that reflects the market’s expectation of profitability for the company being valued.
The implementation of this method relies on the projections of PHÉRÉCYDES’ management.
These management projections take into account the capacity of PHÉRÉCYDES to finance its development on a stand-alone basis given the strong cash consumption expected at the beginning of the period.
These projections assume that the first treatment developed by PHÉRÉCYDES will be launched in 2026, and that it will rapidly gain market share after that date, generating significant revenue. The second treatment is expected to be launched on the market in 2027.
17

The terminal value has been calculated in 2033, on the basis of the latest cash flow.
The discount rate used is 17.5%, reflecting the execution risk level of management’s projections, and the perpetual growth rate is 1.1%.
On these bases, the discounted cash flow method results in a value of €2.62 per PHÉRÉCYDES share as the central value, i.e. an overall value of €19,187,229.
| (iii) | Reference to analysts’ price targets |
The analysis of financial analysts’ price targets does not represent a valuation method as such, but rather summarizes opinions on the value. This reference consists of observing the value of a company on the basis of the price targets published by financial analysts.
PHÉRÉCYDES is subject to limited monitoring by a single analyst, PORTZAMPARC.
The last price target published before the announcement of the operation was €7.40 per share, i.e. an overall value of €54,192,938.
| 2.5.2 | Assessment of the overall value by the merger auditor |
In order to assess the overall value of the contribution, we performed our own valuation work using a multi-criteria approach based on:
| • | primarily, reference to the PHÉRÉCYDES stock price and the discounted cash flow method; |
| • | for information purposes, reference to analysts’ price targets. |
| 2.5.2.1 | Reference to the stock price |
In order to implement this approach, we have used the stock price of PHÉRÉCYDES as of January 19, 2023, corresponding to the date of signature of the letter of intent (“LOI”) and of the determination of the parity as part of the Merger; and as of February 15, 2023, corresponding to the date of the operation being announced to the market (after the stock market close).
Our analyses were conducted on the basis of the spot price and the 1-month, 60-day, 3-month, 6-month and 12-month VWAP calculated at the two dates mentioned above.
18

| 2.5.2.2 | Discounted cash flows (DCF) |
We have been provided with the projections of PHÉRÉCYDES’ management, which take into account PHÉRÉCYDES’ ability to finance its development on a stand-alone basis given the expected cash consumption at the beginning of the period.
In accordance with our procedures, we have reviewed the main assumptions used to estimate future cash flows and have carried out our own valuation.
| Discount | rate and perpetual growth rate: |
The weighted average cost of capital to discount the future cash flows of PHÉRÉCYDES takes into account a risk-free rate based on the TEC 10 OAT, an equity market risk premium, an unlevered beta of identified comparables and a specific risk premium to reflect the risk related to the development and obtaining of marketing authorizations for the treatments currently in phase I or II.
In terms of perpetual growth, we have used a rate consistent with the long-term inflation assumptions for France.
Net debt position:
PHÉRÉCYDES’ adjusted net debt has been determined on the basis of the company’s financial statements as at December 31, 2022.
| Summary: |
On this basis, taking into account its debt position as at December 31, 2022, the overall value of the contribution is in the middle of PHÉRÉCYDES’ share valuation range resulting from our analysis.
| 2.5.2.3 | Reference to analysts’ price targets |
To implement this approach, which is presented for information purposes, we have used the last price target published by the only analyst monitoring PHÉRÉCYDES.
| 3. | SUMMARY – KEY POINTS |
The contribution value of €16,537,386 was determined on the basis of the actual value of the assets and liabilities making up the net assets of the Acquired Company.
19

The range of PHÉRÉCYDES values, derived from our multi-criteria approach, supports the contribution value used by the parties, it being specified that:
| • | The contribution value was determined, in particular, by referring to the market spot price of PHÉRÉCYDES on January 19, 2023; |
| • | The contribution value falls within the range of the volume-weighted average stock price on January 19, 2023; |
| • | The contribution value falls in the middle of our range of values resulting from the application of the discounted cash flow (DCF) valuation approach, which we consider to be the most appropriate in view of PHÉRÉCYDES’ specificities. |
Our value estimates are based on a stand-alone approach and do not include any of the synergies anticipated by the parties.
We have confirmed with PHÉRÉCYDES and ERYTECH that there were no elements that significantly called into question the data provided to us and that we used in the context of our work.
The results of our valuation work and the sensitivity analyses of certain structuring parameters (discount rate, perpetual growth rate and operating parameters) do not call into question the value of the net assets of the Acquired Company used as the contribution value.
It should be noted that prior to the completion of the Merger, some shareholders of the Acquired Company undertook to contribute 827,132 ordinary shares of PHÉRÉCYDES to ERYTECH. Under the terms of the Merger Agreement, it is stipulated that the PHÉRÉCYDES shares that will be held by the Acquiring Company following this contribution in kind will not be subject to the exchange ratio. Consequently, the Acquiring Company will only increase its capital for the portion of assets corresponding to the shares that it does not hold in the Acquired Company on the Completion Date, i.e. €14,757,430.84 corresponding to the value of the contributions for which consideration will be paid.
20
| 4. | CONCLUSION |
On the basis of our work and as of the date of this report, we are of the opinion that the value of the contributions, amounting to €16,537,386, is not overstated. It should be noted that only the portion of the net assets corresponding to the shares of the Acquired Company not held by the Acquiring Company on the Completion Date, i.e. €14,757,430.84, will give rise to the capital increase. As such, the value of the contributions for which consideration will be paid, amounting to €14,757,430.84, is at least equal to the amount of the capital increase of the Acquiring Company plus the merger premium.
Signed in Paris, France on May 15, 2023
The Merger Auditor
FINEXSI EXPERT & CONSEIL FINANCIER
Christophe Lambert
Statutory Auditors
Members of the Paris Regional Association of Statutory Auditors (Compagnie Régionale de Paris)
103
ANNEX 3. TEXT OF THE RESOLUTIONS OF THE GENERAL MEETINGS OF THE SHAREHOLDERS OF ERYTECH AND PHERECYDES
| 17. | APPROVAL OF THE MERGER; APPROVAL OF THE TERMS AND CONDITIONS OF THE MERGER AGREEMENT; APPROVAL OF THE CONTRIBUTIONS, THEIR VALUATION, AND THEIR REMUNERATION |
The General Shareholders’ Meeting, voting under the rules of quorum and majority required for Extraordinary Shareholders’ Meetings, ruling in accordance with Articles L. 236-1 to L. 236-6 and L. 236-8 to L. 236-15 of the French Commercial Code, after having reviewed :
- the Board of Directors’ report,
- the report prepared by Finexsi, a public limited company (société anonyme) having its registered office located 14 rue de Bassano 75016 Paris, registered with the Paris Trade and Companies Register under number 412 029 357, represented by Christophe Lambert, Merger appraiser appointed by an ordonnance of the President of the Lyon Commercial Court on February 28, 2023 (the “Merger Appraiser”), on the terms and conditions of the Merger, the value of the contributions, their valuation and their remuneration.
- the merger agreement and its annexes (the “Merger Agreement”) concluded by private deed dated May 5, 2023, between the Company and Pherecydes Pharma, a French société anonyme with a share capital of EUR 5,859,476, having its registered office at 22 boulevard Benoni Goullin, 44200 Nantes, registered with the Nantes Trade and Companies Register under number 493 252 266 (“Pherecydes”), under the terms of which it is agreed that Pherecydes will contribute to the Company, by way of merger, all of its assets and liabilities in accordance with the provisions of Articles L. 236-1 to L. 236-6 of the French Commercial Code (the “Merger”),
subject to the fulfillment or waiver of the conditions precedent set forth in Article 16 of the Merger Agreement (the “Conditions precedent”),
- the exemption document serving as a prospectus exemption in the event of a merger and its annexes dated 23 May 2023 (the “Exemption Document”),
- the positive opinion of the Company’s Works Council dated March 20, 2023, and
- the text of the resolutions submitted to the general shareholders’ meeting of Pherecydes convened this day in order to approve the Merger Agreement, the Merger, and the dissolution without liquidation of Pherecydes,
104
approves without any restrictions or limitations, in all its provisions, the Merger Agreement, and in particular :
| • | the total fair value of the net assets contributed by Pherecydes amounting to EUR 16,537,386 for a total number of 7,939,179 existing ordinary shares, it being specified that this fair value has been determined in accordance with the valuation methods set forth in Annex 14.1 of the Merger Agreement, and the fair value per ordinary share, at EUR 2.29, |
| • | the exchange ratio, agreed by mutual agreement, is consequently established at 4 ordinary shares of Pherecydes for 15 ordinary shares of the Company, |
| • | the terms of remuneration of the Merger consisting, on the one hand, in the assumption by the Company of the liabilities of Pherecydes, including in particular those listed in the Merger Agreement, and, on the other hand, in the allocation to the shareholders of Pherecydes, of a total number of 26,575. 893 ordinary shares of the Company with a par value of EUR 0,10 each, together with a balancing payment of a total amount of EUR 0,42, fully paid up, to be created as an increase of the share capital of the Company, it being specified that the final number of new shares to be issued and, accordingly, the nominal amount of the resulting share capital increase will be adjusted by operation of law according to the exact number of Pherecydes shares to be remunerated under the Merger, |
| • | the fact that the Company will not proceed to any compensation of any balancing payment and that the Pherecydes shareholders expressly waive the payment of any balancing payment, |
| • | the fact that the final completion of the Merger will be legally effective on the date of the final completion of the latest of the Conditions Precedent (the “Completion Date”), |
| • | that the Merger will be effective, from a tax and accounting perspective, as of January 1, 2023, |
acknowledges :
| • | the transfer of all of Pherecydes’ assets and liabilities to the Company in the context of the Merger, |
| • | the contribution by Pherecydes of all its assets, rights and obligations and, in particular, the valuation of the said contribution, which is established, in accordance with the provisions of Autorité des Normes Comptables (ANC) regulation no. 2014-03 relating to the general chart of accounts of June 5, 2014, as last amended by ANC regulation no. 2022-01 of March 11, 2022, at its real value, i.e., the sum of EUR 16,537,386, |
| • | the fact that the difference between the net value of the assets contributed by Pherecydes and remunerated by the Absorbing Company (i.e. EUR 14,757,430.84) and the nominal amount of the capital increase of the Company with a balancing payment of EUR 0,42 (EUR 2,657,589.72), will be booked as a liability on the balance sheet of the Company, in an account entitled “Merger Premium”, to which the rights of the existing and new shareholders of the Company will be attached, |
105
| • | the fact that, in accordance with the provisions of article L. 236-3 of the French Commercial Code, neither the exchange of the ordinary shares of Pherecydes held by the Company as of the Completion Date, nor the exchange of the ordinary shares of Pherecydes held in treasury as of the Completion Date will be carried out, as they will be cancelled by operation of law following the completion of the Merger, |
| • | the fact that fractional rights will not be negotiable or transferable and that, consequently, in accordance with the provisions of articles L. 228-6-1 and R. 228-12 of the French Commercial Code, when the number of shares of the Company to which a shareholder of Pherecydes is entitled does not correspond to a whole number of shares of the Company, the shareholder will receive the number of shares of the Company immediately below, plus of a cash balance resulting from the price at which the shares of the Company corresponding to the fractional shares will have been sold by the financial intermediaries, within thirty days as from the latest of the dates of registration, in the account of the Pherecydes shareholders, of the whole number of shares of the Company allocated, |
| • | that the new ordinary shares issued by the Company will be fully paid up on the Completion Date and assimilated to the existing ordinary shares, that they will have the same rights and will be subject to all the provisions of the Company’s bylaws, and that they will be issued with dividend rights and will be entitled to any distributions made as from their issue date, |
| • | that the new ordinary shares issued by the Company will be the subject to an application for admission to trading on the regulated market of Euronext in Paris and that they will be immediately assimilated to the existing shares of the Company, already traded on Euronext Paris and negotiable, as from their date of admission, on the same trading line as these shares under the same ISIN code FR0011471135, |
acknowledges the Company’s obligations following the transfer, in accordance with (i) the provisions of Article L. 225-197-1 and L. 228-98 to L. 228-106 of the French Commercial Code and (ii) the Merger Agreement, of the commitments of Pherecydes with respect to the free allocation of ordinary shares by Pherecydes (the “AGA”) and the issuance of founder’s share warrants issued by Pherecydes prior to the completion of the Merger (the “BSPCE”), and, in particular :
| • | acknowledges that, as from the Completion Date, the Company will be fully substituted to Pherecydes in its obligations towards the beneficiaries of the AGA and the holders of the BSPCE, |
106
| • | decides to apply the exchange ratio set forth in article 14.1 of the Merger Agreement according to the following terms: the number of ordinary shares of Pherecydes to which each beneficiary would be entitled in the case of the same grant plan shall correspond to the number of ordinary shares of the Company to which such beneficiary would have been entitled pursuant to this plan multiplied by the merger ratio applicable to the shareholders set forth in article 14.1 of the Merger Agreement, the number thereby obtained being rounded down to the closest whole number, |
| • | as a consequence for the BSPCE holders: |
| • | acknowledges that the BSPCE’s will give right, in case of exercise, to the subscription of a maximum number of 2,207,774 ordinary shares of the Company, |
| • | acknowledges that the Merger Appraiser has issued an opinion on this maximum number of ordinary shares of the Company, |
| • | acknowledges that the approval of the Merger proposal by the shareholders of the Company pursuant to this resolution entails the waiver by the latter of their preferential subscription right to the ordinary shares that would be issued upon exercise of the BSPCEs to the benefit of the holders of BSPCEs, |
| • | authorizes the issue of the 2,207,774 ordinary shares of the Company likely to result from the exercise of the BSPCEs to the beneficiaries of the BSPCEs, representing a capital increase of a maximum nominal amount of EUR 220,777.40, and |
| • | grants full powers to the Board of Directors to record the final completion of the resulting increases in the Company’s share capital and, for this purpose (i) to receive the subscriptions for new ordinary shares and the corresponding payments and to deposit them with the Company’s bank and (ii) more generally, to take all useful measures and carry out all formalities necessary for the final completion and publication of the said increases in the Company’s share capital resulting from the exercise of the BSPCEs, and to make the corresponding amendments to the bylaws, and |
| • | as a consequence for the beneficiaries of the AGA: |
| • | acknowledges that the 43,892 ordinary shares of Pherecydes granted free of charge to the beneficiaries of AGAs and not definitively acquired on the Completion Date will give right, upon their definitive acquisition, to a maximum number of 164,595 ordinary shares of the Company, |
| • | waives, insofar as is necessary, the preferential subscription right to the ordinary shares that may be issued by the Company as a result of the definitive acquisition of these instruments in accordance with the terms of the AGA plans, it being specified that this decision entails, insofar as is necessary, the waiver by the shareholders in favor of the AGA beneficiaries of that part of the reserves, profits or premiums that will be used in the event of the issue of new shares at the end of the vesting period, for the realization of which all powers are delegated to the Board of Directors, and |
| • | grants full powers to the Board of Directors to record the definitive acquisition by the beneficiaries of the AGAs, at the end of the vesting period, of the ordinary shares of the Company concerned, grants full powers to the Board of Directors to record the final completion of the resulting increases in the Company’s share capital and, to this end, to take all necessary measures and carry out all formalities required for the final completion and publicity of the said increases in the Company’s share capital and to make the corresponding amendments to its bylaws. |
107
| 18. | INCREASE IN THE COMPANY’S SHARE CAPITAL AS MERGER CONSIDERATION |
The General Shareholders’ Meeting, voting under the rules of quorum and majority required for Extraordinary Shareholders’ Meetings, and ruling in accordance with Articles L. 236-1 to L. 236-6 of the French Commercial Code, subject to the conditions precedent of (i) the approval by this Shareholders’ Meeting of the previous resolution and (ii) the fulfillment of the Conditions Precedent
after having reviewed :
| • | the Board of Directors’ report, |
| • | the Merger Appraiser’ report on the terms and conditions of the Merger, the value of the contributions, their valuation, and their remuneration |
| • | the Merger Agreement, and |
| • | the Exemption Document, |
decides :
| • | the issuance, as consideration for the Merger, of a total of 26,575,893 new ordinary shares with a par value of EUR 0,10 each, with a balancing payment of a total amount of EUR 0,42, fully paid up and assimilated to the already existing ordinary shares, giving right to any distribution made as from their date of issuance and subject to all the provisions of the Company’s by-laws, representing an increase in the share capital of the Company of an amount of EUR 2,657,589.30 to increase it from EUR 3,412,029.80 to EUR 6,069,619.10 euros, it being specified that the definitive number of new shares to be issued and consequently the nominal amount of the resulting capital increase will be adjusted by operation of law according to the exact number of Pherecydes shares to be remunerated pursuant to the Merger, |
| • | that the difference between the net value of the assets contributed by Pherecydes and remunerated by the Absorbing Company (i.e. EUR 14,757,430.84) and the nominal amount of the capital increase of the Company with a balancing payment of EUR 0,42 (i.e. EUR 2,657,589.72), i.e. the sum of EUR 12,099,841.12, represents the amount of the Merger Premium on which the rights of the existing and new shareholders will be based and will be accounted within the liabilities of the balance sheet of the Company, under an account “Merger Premium”, |
108
authorizes the Board of Directors to :
| • | charge to the Merger Premium account all costs and expenses of any nature whatsoever resulting from the completion of the Merger, including all amounts necessary for the assumption of the liabilities of Pherecydes by the Company, it being specified that the balance of the Merger Premium may be allocated at any time in accordance with the applicable rules decided by the shareholders’ meeting, and |
| • | to deduct, as the case may be, from the Merger Premium any omitted or undisclosed liabilities relating to the transferred assets. grants all powers to the Board of Directors of the Company, with the right to sub-delegate in accordance with the legal and regulatory conditions, in order to implement this resolution, and in particular : |
| • | to acknowledge the completion of the Conditions Precedent (or the waiver of such Conditions Precedent) and, as a consequence, to acknowledge the final completion of the Merger, |
| • | to acknowledge the definitive number of new shares of the Company to be issued as consideration for the Merger and, accordingly, the definitive amount and completion of the capital increase on the Completion Date, as well as the definitive amount of the Merger Premium and to decide on the amendments to the by-laws resulting from the definitive completion of the Merger, |
| • | to sign the declaration of regularity and conformity provided for in Article L. 236-6 of the Commercial Code, |
| • | to carry out all formalities required for the admission of the new ordinary shares of the Company to trading on compartment C of the regulated market of Euronext Paris |
| • | and, more generally, to proceed with all observations, declarations, or communications, to draw up all reiterative, confirmatory, corrective or supplementary deeds, and to take all measures, sign all documents, deeds or contracts and carry out all formalities or steps useful or necessary for the final completion of the Merger. |
| 19. | ACKNOWLEDGEMENT OF THE FINAL COMPLETION OF THE MERGER AND DISSOLUTION OF PHERECYDES |
The General Shareholders’ Meeting, voting under the rules of quorum and majority required for Extraordinary Shareholders’ Meetings, subject to the approval by this General Meeting of the 17th and 18th resolutions and after having reviewed :
| • | the Board of Directors report, |
| • | the statutory auditor’ report of the Merger, on the terms and conditions of the Merger, the value of the contributions, their valuation and their remuneration, |
| • | the Merger Agreement, and |
| • | the Exemption Document, |
109
grants all powers to the Board of directors, with the right of sub-delegation, in order to :
| • | acknowledge (i) the completion of the Conditions Precedent (or the waiver of such Conditions Precedent) and (ii) the completion of the Merger, with all its consequences, including the dissolution without liquidation of Pherecydes as a result of the Merger; |
| • | to proceed with all observations, communications and formalities that may be necessary for the purposes of the completion of the Merger, |
grants all powers to the Chief Executive Officer, with the option to sub-delegate such powers, in order to (i) carry out all necessary steps for the creation of the new shares of the Company and their listing on the regulated market of Euronext Paris and (ii) prepare and sign the declaration of compliance provided for in article L. 236-6 of the French Commercial Code and (iii) more generally, to carry out all findings, communications and formalities necessary for the completion of the Merger.
| 20. | CONSEQUENTIAL AMENDMENT OF ARTICLES 6 (“ESTABLISHMENT OF THE CAPITAL”) AND 7 (“CAPITAL STOCK”) OF THE COMPANY’S BYLAWS |
The General Shareholders’ Meeting, voting under the rules of quorum and majority required for Extraordinary Shareholders’ Meetings, after having reviewed the Board of Directors’ report,
decides, subject to the condition precedent of the final completion of the Merger, to amend Articles 6 (“Establishment of the capital”) and 7 (“Capital stock”) of the Company’s bylaws, which shall henceforth read as follows as from the completion of the Merger:
“ARTICLE 6. ESTABLISHMENT OF THE CAPITAL
[...]
Under the terms of the deliberations of the Combined General Meeting of June 23, 2023, the share capital was increased by EUR 2,657,589.30 to bring it from EUR 3,412,029.80 to EUR 6,069,619.10, through the issue of 26,575,893 shares with a par value of EUR 0,10 each, with a balancing payment of EUR 0,42.
ARTICLE 7. SHARE CAPITAL
The share capital is set at the sum of six million sixty-nine thousand six hundred and nineteen euros and ten cents (€ 6,069,619.10).
110
It is divided into sixty million six hundred and ninety-six thousand one hundred and ninety-one (60,696,191) shares with a par value of ten cents (0.10) euro each, all of the same class and fully paid up.”
| 21. | MODIFICATION OF THE COMPANY’S CORPORATE NAME AS FROM THE DATE OF COMPLETION; CORRESPONDING AMENDMENT OF ARTICLE 2 (“NAME”) OF THE COMPANY’S BY-LAWS |
The General Shareholders’ Meeting, voting under the rules of quorum and majority required for Extraordinary Shareholders’ Meetings, after having reviewed the Board of Directors’ report, the Merger Agreement and the Exemption Document, subject to the final completion of the Merger,
decides, in accordance with the provisions of the Merger Agreement, to change the corporate name of the Company to “Phaxiam Therapeutics”,
decides as a consequence to amend Article 2 (“Name”) of the by-laws of the Company, which shall henceforth read as follows
“ARTICLE 2. NAME
The Company’s name is :
Phaxiam Therapeutics
In all deeds and documents created by the Company and intended for third parties, its name shall be immediately preceded or followed by the words “Société Anonyme” or the abbreviation “SA” and a declaration of its capital stock, head office, and registration number in the trade and companies register.”
| 22. | REMOVAL OF THE CHAIRMAN’S CASTING VOTE AT MEETINGS OF THE BOARD OF DIRECTORS; AMENDMENT OF THE AGE LIMIT FOR OBSERVERS; CONSEQUENTIAL AMENDMENT OF ARTICLES 18 (“ORGANIZATION OF THE BOARD”) AND 19 (“BOARD DELIBERATIONS”) OF THE COMPANY’S BY-LAWS |
The General Shareholders’ Meeting, voting under the rules of quorum and majority required for Extraordinary Shareholders’ Meetings, after having reviewed the Board of Directors’ report, the Merger Agreement, and the Exemption Document, subject to the final completion of the Merger,
resolves to
| (i) | to remove the casting vote of the Chairman of the Board of Directors in the event of a tie; and |
| (ii) | to remove the maximum age limit for observers, |
111
decides, as a consequence, to amend Articles 18 (Organization of the Board) and 19 (Board Deliberations) of the Company’s by-laws, which will henceforth read as follows
“ARTICLE 18. ORGANIZATION OF THE BOARD
[...]
The Board may designate, within a maximum limit of two, one or more observers who are natural persons, directors or otherwise, without age limit.
[...].
ARTICLE 19. BOARD DELIBERATIONS
[...]
In the event of a tie, the Chairman of the meeting shall not have a casting vote.”
112
PHERECYDES PHARMA
French Société anonyme with a Board of Directors, with a share capital of €7,939,179
Registered office: Nantes Biotech, 22, Boulevard Benoni Goullin - 44200 Nantes (France)
493 252 266 RCS Nantes
(the “Company”)
TEXT OF THE RESOLUTIONS PROPOSED TO THE
ANNUAL ORDINARY AND EXTRAORDINARY GENERAL MEETING OF
SHAREHOLDERS OF JUNE 23, 2023
AGENDA
Falling under the powers of the Ordinary General Meeting:
| • | Presentation of the Board of Directors’ reports |
| • | Reading of the Statutory Auditor’s reports |
| • | Approval of the financial statements for the financial year ended December 31, 2022 (1st resolution) |
| • | Allocation of the earnings for the financial year ended December 31, 2022 (2nd resolution) |
| • | Approval of the agreements referred to in Articles L. 225-38 et seq. of the French Commercial Code (3rd resolution) |
| • | Authorization to be granted to the Board of Directors for the purchase by the Company of its own shares (4th resolution) |
Falling under the powers of the Extraordinary General Meeting:
| • | Approval of the proposed merger by absorption of Pherecydes Pharma by Erytech Pharma (5th resolution) |
| • | Dissolution without liquidation of Pherecydes Pharma (6th resolution) |
| • | Authorization to be granted to the Board of Directors to reduce the share capital by cancelling treasury shares (7th resolution) |
| • | Delegation of authority to the Board of Directors to increase the capital by incorporation of premiums, reserves, profits or others (8th resolution) |
| • | Authorization to the Board of Directors to grant subscription and/or purchase options for ordinary shares (the “Options”) with cancellation of the preferential subscription right of shareholders in favour of a category of persons (9th resolution) |
113
| • | Delegation of authority to the Board of Directors to issue and allocate share warrants for the subscription of ordinary shares (the “Warrants”) with cancellation of the preferential subscription right in favour of a category of persons (10th resolution) |
| • | Delegation of authority to the Board of Directors to issue and allocate founders’ warrants (“BSPCE” or “Founders’ Warrants”) with cancellation of the preferential subscription right in favour of a category of persons (11th resolution) |
| • | Authorization granted to the Board of Directors to proceed with the free allocation of shares, existing or to be issued (the “Free Shares”), with cancellation of the preferential subscription right of shareholders in favour of a category of persons (12st resolution) |
| • | Setting of overall limits of amount of issuances carried out pursuant to the authorizations to grant |
| • | Options and Free Shares and to delegations to issue Warrants and Founders’ Warrants (13th resolution) |
| • | Delegation to the Board of Directors to carry out a share capital increase by issuing shares or securities giving access to the share capital, reserved for members of a company savings plan with cancellation of the preferential subscription right in favour thereof (14th resolution) |
| • | Powers for formalities (15th resolution); |
RESOLUTIONS SUBMITTED TO THE ORDINARY GENERAL MEETING
FIRST RESOLUTION
Approval of the financial statements for the financial year ended December 31, 2022
The General Meeting,
ruling under the conditions of quorum and majority for ordinary general meetings,
having reviewed (i) the reports of the Board of Directors; and (ii) the report of the Statutory Auditor on the financial statements for the financial year ended December 31, 2022;
approves the transactions reflected in the financial statements and summarized in these reports, as well as the financial statements for the financial year ended December 31, 2022, as presented, which show a loss of €4,854,342.48,
notes that no expenses and costs referred to in Articles 39-4 and 39-5 of the French General Tax Code were recorded during the year.
SECOND RESOLUTION
Allocation of the earnings for the financial year ended December 31, 2022
The General Meeting,
ruling under the conditions of quorum and majority of ordinary general meetings,
having reviewed (i) the reports of the Board of Directors; and (ii) the report of the Statutory Auditor on the financial statements for the financial year ended December 31, 2022;
approves the proposal of the Board of Directors and decides to allocate the loss of €4,854,342.48 for the financial year ended December 31, 2022, in full to the “Retained Earnings” account,
114
acknowledges, in accordance with the provisions of article 243 bis of the French General Tax Code, that no distribution of dividends occurred in the last three financial years.
THIRD RESOLUTION
Approval of the agreements referred to in Articles L. 225-38 et seq. of the French Commercial Code
The General Meeting,
ruling under the conditions of quorum and majority for ordinary general meetings,
having read the Statutory Auditor’s special report on the agreements referred to in Articles L. 225-38 et seq. of the French Commercial Code,
approves the terms of said report and records the absence of new agreement.
FOURTH RESOLUTION
Authorization to be granted to the Board of Directors for the purchase by the Company of its own shares
The General Meeting,
ruling under the conditions of quorum and majority for ordinary general meetings,
having reviewed the report of the Board of Directors,
pursuant to Article L. 22-10-62 of the French Commercial Code,
authorizes the Board of Directors, with the right of sub-delegation under the conditions provided by law, to acquire a number of shares of the Company which may not exceed 10% of the total number of shares comprising the share capital on the date of repurchase by the Company; it being specified that when the shares are purchased under a liquidity agreement, the number of shares taken into account in the calculation of the 10% limit corresponds to the number of shares purchased less the number of shares resold during the term of the authorization;
resolves that the acquisition, sale or transfer of these shares may be carried out by any means, from time to time, in particular on the market or over the counter, including by acquisition or block sales, public offers, by using optional or derivative mechanisms, under the conditions provided by the market authorities and in compliance with applicable regulations;
decides that the maximum purchase price per unit for shares (excluding fees and commission) shall not exceed €10, subject to adjustments intended to take into account the impact of new transactions on the Company’s share capital, namely modification of the nominal price of shares, increase of share capital by incorporation of reserves, free allocation of shares, share split or reverse stock split, distribution of reserves or of any other assets, amortisation of capital, or of any other transaction on shareholders’ equity, capped at a maximum theoretical amount that may be paid by the Company under this authorization equal to €7,939,179 on the basis of the maximum percentage of 10% of the total number of shares making up the share capital on the date of the General Meeting;
resolves that this authorization to operate on the Company’s own shares is granted with the purpose to:
| • | ensuring the liquidity of the Company’s securities through an investment service provider acting independently within the framework of a liquidity agreement in accordance with accepted practice under regulations; and/or |
115
| • | honouring obligations under share purchase option plans, free allocations of shares, employee savings plans or other share allocations to employees and directors of the Company or companies related to it, as well as carrying out all hedging transactions relating to these transactions, under the conditions and pursuant to the provisions of applicable laws and regulations; and/or |
| • | delivering shares by virtue of the exercise of rights attached to securities giving access to the share capital, as well as carrying out all hedging transactions relating to these transactions under the conditions and pursuant to the provisions of applicable laws and regulations; and/or |
| • | purchasing shares for retention and future delivery in exchange or in payment in relation to any external growth, merger, demerger or asset contribution transactions; and/or |
| • | cancelling all or part of the securities so repurchased, subject to the adoption of the 7th resolution below and then, under the terms indicated therein; and/or |
| • | carrying out any transaction pursuant to regulations in force; and/or |
| • | more generally, achieving any purpose that may be authorized by law or any market practice that may be accepted by market authorities, it being specified that, in such a case, the Company would inform its shareholders through a press release; |
resolves that the number of shares acquired by the Company for retention and future delivery for payment or exchange in relation to a merger, demerger or asset contribution transaction may not exceed 5% of its share capital;
decides that the Board of Directors shall have all the powers to implement this authorization, with the option of sub-delegation under the conditions provided by law, in particular whether to deem it appropriate to launch a buy-back program and to determine the terms and conditions thereof, to place all stock market orders, to execute all deeds of sale or transfer, to enter into all agreements, all liquidity agreements, all options agreements, to proceed with all statements with the French Financial Markets Authority and any other body, and all necessary formalities, in particular to allocate or reallocate the acquired shares to the various formalities, and, as a general matter, to do everything necessary;
resolves that this authorization shall be valid for a period of eighteen (18) months from the date of this meeting;
resolves that this delegation of authority shall deprive of its effects any previous delegation with the same subject matter, as the case may be to the extent of its unused part
RESOLUTIONS SUBMITTED TO THE EXTRAORDINARY GENERAL MEETING
FIFTH RESOLUTION
Approval of the proposed merger by absorption of Pherecydes Pharma by Erytech Pharma
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings,
after taking note of:
| • | the report of the Board of Directors of the Company; |
| • | the executed merger agreement, including its annexes (the “Merger Agreement”), established by private deed on May 15, 2023 between Erytech Pharma, a French société anonyme (limited liability company), whose registered office is located at 60, Avenue Rockefeller, 69008 Lyon (France), and registered with the Lyon Trade and Companies Register under number 479 560 013 (“Erytech”), as the Absorbing Company, and the Company, as the Absorbed Company, and relating to the proposed merger of the Company into Erytech (the “Merger”); |
116
| • | the report prepared by Finexsi, a public limited company with its registered office at 14 rue de Bassano, 75016 Paris (France), registered with the Paris Trade and Companies Register under number 412 029 357, in the person of Mr. Christophe Lambert, an independent merger appraiser (“commissaire à la fusion”) appointed by order of the President of the Lyon Commercial Court on February 28, 2023 (the “Merger Appraiser”), on the terms and conditions of the merger, the value of the contributions, their valuation and their remuneration (the “Merger Appraiser’s Report”); |
| • | the exemption document, which exempts the preparation of a prospectus in the event of a merger, and its annexes (the “Exemption Document”); |
approves the Merger Agreement, in all its provisions, under the terms of which it is agreed that the Company contributes to Erytech, by way of merger, all the assets and liabilities composing its assets, and in particular:
| • | the universal transfer of the Company’s assets to Erytech; |
| • | the total fair market value of the net assets contributed by the Company amounting to €16,537,386 for a total number of 7,939,179 existing ordinary shares, it being specified that this fair market value has been determined in accordance with the valuation methods set forth in Appendix 14.1 of the Merger Agreement, and the fair market value per ordinary share, at €2.29; |
| • | the fact that the exchange ratio, as agreed upon, is consequently 4 ordinary shares of the Company for 15 ordinary shares of Erytech; |
| • | the terms of remuneration of the Merger consisting, on the one hand, in the taking over by Erytech of the liabilities of the Company, including those listed in the Merger Agreement, and, on the other hand, in the allocation to the shareholders of the Company of a total number of 26,575,893 fully paid-up ordinary shares of Erytech with a nominal value of ten cents (€0.10) each, to be issued as a share capital increase by the Absorbing Company, it being specified that the final number of new shares to be issued and, accordingly, the nominal amount of the resulting capital increase will be adjusted by operation of law according to the exact number of Pherecydes shares to be remunerated under the Merger; |
| • | the determination of the date of legal completion of the Merger and of the automatic dissolution of the Company on the date of the final fulfilment of the last of the conditions precedent set forth in Article 16 of the Merger Agreement (the “Completion Date”); |
| • | subject to the adoption of this resolution and of the 6th resolution below, the fact that the definitive completion of the Merger will occur on the Completion Date at 11:59 p.m.; |
| • | the fact that the Merger will take effect, from a fiscal and accounting point of view, on January 1st, 2023; |
notes that:
| • | in accordance with the provisions of Article L. 236-3 of the French Commercial Code, there will be no exchange of Pherecydes ordinary shares held by Erytech as of the Completion Date, nor any exchange of Pherecydes ordinary shares held by Pherecydes (treasury shares) as of the Completion Date, all of which will be cancelled, as of right, on the Completion Date of the Merger; |
| • | the rights forming fractional shares (“rompus”) will not be negotiable or transferable and that, consequently, in accordance with the provisions of articles L. 228-6-1 and R. 228-12 of the French Commercial Code, when the number of Erytech shares to which a shareholder of the Company is entitled does not correspond to a full number of Erytech shares, the shareholder will receive the number of Erytech shares immediately below, plus for the entire balance, a cash balance resulting from the price at which the Erytech shares corresponding to the fractional shares will have been sold, paid by the financial intermediaries referred to in Article 14.4 of the Merger Agreement; |
117
| • | the new ordinary shares issued by Erytech will be, at the Completion Date, fully paid up and assimilated to the existing ordinary shares, they will have the same rights and will be subject to all the provisions of Erytech’s by-laws, will be issued with current dividend rights and will give the right to any distribution paid as from their issue date; |
| • | the new ordinary shares issued by Erytech will be the subject of an application for admission to trading on the regulated market of Euronext Paris and that they will be immediately assimilated to the existing shares of Erytech, already traded on Euronext Paris and negotiable, as of their date of admission, on the same quotation line as these shares under the same ISIN Code FR0011471135, |
grants all powers to the Board of Directors of the Company, with the option to delegate such powers within the limits authorized by law, in order to carry out all statement, communications and formalities that may be necessary for the purposes of the completion of the Merger.
SIXTH RESOLUTION
Dissolution without liquidation of Pherecydes Pharma
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings,
after taking note of:
| • | the report of the Board of Directors of the Company; |
| • | the executed Merger Agreement; |
| • | the Merger Appraiser’s Report; |
| • | the Exemption Document; |
resolves, subject to the adoption of the foregoing resolution, that the Company will be dissolved by operation of law without liquidation, on the Completion Date, at 11:59 p.m.
SEVENTH RESOLUTION
Authorization to be granted to the Board of Directors to reduce the share capital by cancelling treasury shares
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings,
having read (i) the report of the Board of Directors; and (ii) the Statutory Auditor’s report;
subject to the adoption of the 4th resolution above,
authorizes the Board of Directors, pursuant to Article L. 22-10-62 of the French Commercial Code, with the option of sub-delegation in the conditions provided by law, to cancel, on one or more occasions, up to a maximum of 10 % of the amount of the share capital per period of twenty-four (24) months, all or part of the shares acquired by the Company and to proceed, for the due amount, with a reduction in the share capital, it being specified that this limit applies to an amount of the share capital which, where applicable, shall be adjusted to take into account the transactions that would affect it after the date of this meeting;
118
resolves that any excess of the purchase price of the shares over their nominal value shall be charged to the share premium, merger or contribution premium items or to any available reserve item, including the legal reserve, provided that it does not fall below 10% of the Company’s share capital after the capital reduction is executed;
resolves that these transactions may be carried out at any time, including, within the limits permitted by the applicable regulations, during a public offering period for the Company’s securities;
grants all powers to the Board of Directors to proceed with the reduction of the share capital by cancellation of the shares, to determine the final amount of the capital reduction, to set the terms and conditions of the same and to record its completion, to allocate the difference between the book value of the cancelled shares and their nominal amount to all available reserve items and premiums and, more generally, to carry out all acts, formalities or declarations with a view to making the capital reduction(s) that may be carried out under this authorization final and consequently, for the purpose of amending the Company’s bylaws;
resolves that this authorization shall be valid for eighteen (18) months from the date of this meeting;
resolves that this delegation of authority shall deprive of its effects any previous delegation with the same subject matter, where applicable, for the unused part of this latter delegation.
EIGHTH RESOLUTION
Delegation of authority to the Board of Directors to increase the capital by incorporation of
premiums, reserves, profits or other amounts
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings;
having reviewed the report of the Board of Directors;
pursuant to Articles L. 225-130 and L. 22-10-50 of the French Commercial Code,
delegates to the Board of Directors, with the option of sub-delegation under the conditions provided for in the law, its authority to decide one or more capital increases, in the proportions and at the times it will appreciate, in France and abroad, by incorporation into the premium capital, reserves, profits or other whose capitalisation will be legally and statutorily possible and in the form of an allocation of new free shares, an increase in the nominal value of existing shares or joint employment of these two processes, the said shares conferring the same rights as the old shares subject to their date of enjoyment,
decides that the total nominal amount of the share capital increases that may be carried out immediately and/or in the future may not exceed €150,000, amount to be added, as the case may be, to the additional amount of shares to be issued in order to preserve, in accordance with the legal or regulatory provisions and, where applicable, the applicable contractual provisions, the rights of holders of securities or other rights giving access to shares,
decides, in accordance with the provisions of Article L. 225-130 of the French Commercial Code, that in the event of use of this delegation by the Board of Directors, the broken rights will not be negotiable and that the corresponding securities will be sold, the sums resulting from the sale being allocated to the holders of the rights within the time-limit provided for by regulations,
resolves that the transactions referred to in this resolution may be carried out at any time, including during the public offering period for the Company’s securities;
119
resolves that this delegation shall be valid for a period of twenty-six (26) months, starting from this general meeting;
resolves that this delegation of authority shall deprive of its effects any previous delegation with the same subject matter, where applicable, for the unused part of this latter delegation.
NINTH RESOLUTION
Authorization to the Board of Directors to grant share subscription and/or purchase options of
ordinary shares (the “Options”) with cancellation of the preferential subscription right of
shareholders in favour of a category of persons
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings;
having reviewed the reports (i) of the Board of Directors; and (ii) of the Statutory Auditor;
pursuant to Articles L. 225-177 et seq., L. 22-10-56 et seq. and L. 225-129 et seq. of the French Commercial Code,
authorises the Board of Directors, to award options giving right to subscribe for or purchase ordinary shares, during the periods authorized by law, in one or more times, to the employees and/or executives of the Company and companies and economic interest groups related to the Company, under the conditions defined in Article L. 225-180-I of said Code, it being specified that:
| • | the number of options granted under this authorization may not give right to the purchase or subscription of more than 10% of the total number of shares comprising the Company’s share capital, after deducting the subscription or purchase options of the Company granted by the Board of Directors; |
| • | this number will be deducted from the overall ceiling provided for in 13th resolution of this meeting, and the total number of shares that may be subscribed upon exercise of the share subscription options allocated and not yet exercised may never exceed ten percent (10%) of the total number of shares comprising the Company’s share capital; |
specifies that the Board of Directors must comply with the provisions of Article L. 225-186-1 of the French Commercial Code, in order to be able to allocate share subscription or purchase options to the directors of the Company referred to in the fourth paragraph of Article L. 225-185 of the French Commercial Code,
decides that this authorization is entails, in favour of beneficiaries of subscription options, the express waiver by the Company Shareholders of their preferential subscription rights to the shares that would be issued as and when the options are exercised, and that it will be implemented under the conditions and in the manner provided for by the law and regulations in force on the date of the allocation of the options,
decides that the purchase or subscription price per share shall be set by the Board of Directors on the date, on which the option is granted within the limits provided by law and this resolution, without being able to be lower, (i) concerning subscription options, to ninety-five percent (95%) of the average of prices quoted in the twenty trading sessions preceding the day of the Board of Directors’ resolution to allocate the options, rounded up to the higher euro cent, and (ii) in the case of call options, to eighty percent (80%) of the average purchase price of the shares held by the Company, rounded up to the higher euro cent,
120
decides that the price set for the subscription or purchase of the shares, to which the options entitle, may not be modified during the term of the options, it being however specified that, if the Company were to carry out one of the transactions referred to in Article L. 225-181 of the French Commercial Code, it should take the necessary measures to protect the interests of beneficiaries of options under the conditions provided for in Article L. 228-99 of the French Commercial Code,
decides that, in the event of the issuance of new equity securities or new instruments giving access to the share capital and in the event of a merger or demerger of the Company, the Board of Directors may suspend, if applicable, the exercise of the options,
sets the term of validity of the options at ten (10) years from their allocation, it being specified, however, that this period may be reduced by the Board of Directors for beneficiaries resident in a given country insofar as this is necessary in order to comply with the law of that country,
delegates all powers to the Board of Directors, within the limits set above, in order to:
| • | determine the identity of the beneficiaries of share purchase or subscription options and the number of options to be allocated to each of them; |
| • | set the purchase and/or subscription price of the shares, to which the options entitle within the limits of the aforementioned texts, it being specified that the subscription price per share must be higher than the nominal value of the share; |
| • | ensure that the number of share subscription options granted by the Board of Directors is set so that the total number of share subscription options allocated and not yet exercised cannot give right to subscribe to a number of shares exceeding one third of the share capital; |
| • | determine the terms of the stock option plan and set the conditions under which the options will be allocated, including, in particular, the exercise of the options allocated, which may vary according to the holders; it being specified that these conditions may include clauses prohibiting the immediate resale of all or part of the shares issued upon the exercise of options, within the limits set by law; |
| • | proceed with the acquisition of shares of the Company, if any, necessary for the transfer of any shares to which the stock options entitle; |
| • | perform, either by itself or via an agent, all acts and formalities for the purpose of making final the capital increases that may be carried out pursuant to the authorization that is the subject of this delegation; |
| • | attribute, if it considers this necessary, the costs of capital increases against the amount of premiums related to these increases and if necessary, deduct from this amount the sums required to bring the legal reserve up to one-tenth of the new share capital after each increase; |
| • | amend the bylaws accordingly and, generally, do whatever is necessary, |
resolves that the duration of the authorization is set at thirty-eight (38) months from this general meeting;
resolves that this delegation of authority shall deprive of its effects any previous delegation with the same subject matter, where applicable, for the unused part of this latter delegation.
121
TENTH RESOLUTION
Delegation of authority to the Board of Directors to issue and allocate share warrants for the
subscription of ordinary shares (the “Warrants”) with cancellation of the preferential subscription
right in favour of a category of persons
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings;
having reviewed the reports (i) of the Board of Directors; and (ii) of the Statutory Auditor;
pursuant to Articles L. 225-129 et seq., L. 22-10-52, L. 225-135, L. 225-138 and L. 228-92 et seq. of the French Commercial Code,
delegates to the Board of Directors its authority to allocate a maximum number of Warrants, each giving the right to subscribe to one new ordinary share, up to a limit of 15% of the total number of shares comprising the Company’s capital, it being specified that this number will be charged to the overall ceiling provided for in the 13th resolution of this meeting,
decides that the issuance price of a Warrant will be determined by the Board of Directors on the day of the issuance and will not be less than the market value, in accordance with the conclusions of the expert’s report mandated by the Company for the purpose of valuing the subscription price of said Warrant, in accordance with valuation methods applicable to this type of financial instrument,
decides that the subscription price of an ordinary share to be subscribed by exercise of a Warrant will be determined by the Board of Directors at the time of the allocation of the Warrants and must be equal to the weighted average of the prices of the last twenty trading sessions preceding the date of allocation of the said Warrant by the Board of Directors,
decides to cancel, for these Warrants, the preferential subscription right of the shareholders; said Warrants may only be allocated to the following category of beneficiaries: (i) persons holding a corporate office (in the event that the Company is no longer able to issue Founders’ Warrants) or members of any study committee or serving as observer within the Company, (ii) consultants, directors or stakeholders of the Company’s service provider companies that have entered into an agreement for the provision of a Board of Directors or services with the latter in force at the time of use of this delegation by the Board of Directors, and (iii) any person who significantly participates in the scientific or economic development of the Company at the time of use of this delegation by the Board of Directors (the “Warrant Beneficiaries”),
decides, in accordance with the provisions of Article L. 225-138 I of the French Commercial Code, to delegate to the Board of Directors the task of establishing the list of Warrant Beneficiaries and the share of Warrants allocated to each Warrant Beneficiary thus designated,
authorises the Board of Directors accordingly, within the limits of the foregoing, to proceed to issue and allocate the Warrants, in one or more times to each Warrant Beneficiary,
decides to delegate to the Board of Directors for each Warrant Beneficiary, the terms and conditions of exercise of Warrants and, in particular, the issuance price of the Warrant, the subscription price (including issuance premium) of the share, to which each Warrant will entitle (the “Exercise Price”) as set by the Board of Directors under the conditions specified below, and the schedule for exercising the Warrants, it being specified that these will have to be exercised no later than ten (10) years from their issuance and that the Warrants which would not have been exercised at the expiry thereof will lapse as of right,
decides that each Warrant will allow the subscription, under the conditions defined below, of one new ordinary share at an Exercise Price, which will be determined by the Board of Directors on the date of allocation of Warrants, and at least equal to the weighted average of quoted prices in the twenty trading sessions preceding the day of the Board of Directors’ decision to allocate Warrants,
122
decides that the ordinary shares thus subscribed will have to be fully paid up at the time of their subscription, either by payment in cash, or by offsetting against liquid and payable claims,
decides that the new shares delivered to a Beneficiary upon the exercise of its Warrants will be subject to all the statutory provisions and will carry full rights from the first day of the financial year in which they are issued,
decides that the Warrants will be non-transferable. They will be issued in registered form and will be registered in an account,
decides on the issuance of an ordinary shares, to which the exercise of the Warrants issued will entitle,
specifies that pursuant to the provisions of Articles L. 228-91 and L. 225-132 of the French Commercial Code, this decision entails for the holders of Warrants a waiver of their preferential subscription right of ordinary shares, to which the Warrant entitles.
decides, as provided for in Article L. 228-98 of the French Commercial Code, that the Company is authorized, without having to seek the authorization of the holders of the Warrants, to change its form and its corporate purpose,
recalls that in application of Article L. 228-98 of the French Commercial Code, the Company may not change the rules for the distribution of its profits, nor amortize its share capital, nor create preference shares resulting in such change or amortization unless authorized by the issuance agreement or under the conditions provided for in Article L. 228-103 of the French Commercial Code and subject to the necessary arrangements to maintain the rights of holders of securities giving access to capital under the conditions defined in Article L. 228-99 of the French Commercial Code,
authorises the Company to impose on Warrant holders the repurchase or redemption of their rights as provided for in Article L. 228-102 of the French Commercial Code,
decides to grant all powers to the Board of Directors, with the option of sub-delegation within the limits and under the conditions specified above, to implement this delegation, and in order to:
| • | issue and allocate the Warrants and to determine the subscription price, the conditions of exercise and the final terms and conditions of the Warrants in accordance with the provisions of this resolution and within the limits set out in this resolution; |
| • | determine the identity of the Warrant Beneficiaries as well as the number of Warrants to be allocated to each of them; |
| • | set the price of the share, which may be subscribed to in the exercise of a Warrant under the aforementioned conditions; |
| • | note the number of ordinary shares issued as a result of the exercise of the Warrants, carry out the formalities resulting from the corresponding capital increases and amend the bylaws accordingly; |
| • | take steps to ensure the protection of the Warrant holders in the event of a financial transaction involving the Company, in accordance with the legal and regulatory provisions in force; |
| • | in general, take any measures and carry out any formalities useful to this issuance, |
resolves that this delegation shall be valid for a period of eighteen (18) months, starting from the date of this general meeting;
resolves that this delegation of authority shall deprive of its effects any previous delegation with the same subject matter, where applicable, for the unused part of the latter delegation.
123
ELEVENTH RESOLUTION
Delegation of authority to the Board of Directors to issue and allocate founders’ warrants for
entrepreneurs (“BSPCE” or “Founders’ Warrants”) with cancellation of the preferential
subscription right in favour of a category of persons
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings;
having reviewed the reports (i) of the Board of Directors; and (ii) of the Statutory Auditor;
pursuant to Articles L. 225-129 et seq., L. 225-135, L. 225-138 and L. 228-92 et seq. of the French Commercial Code;
noting that the Company meets all of the conditions required for the issuance of Founders’ Warrants under the conditions provided in Article 163 bis G of the French General Tax Code,
delegates to the Board of Directors its authority to allocate a maximum number of Founders’ Warrants, each giving the right to subscribe to one new ordinary share, up to a limit of 15% of the total number of shares comprising the Company’s capital, it being specified that this number will be deducted from the overall limits of amount provided for in the 13th resolution of this meeting,
decides to cancel, for these Founders’ Warrants, the preferential subscription right of the Company shareholders, the said Founders’ Warrants being only allocated to the following category of beneficiaries: employees, officers subject to the tax regime of employees and members of the Board of Directors and of the companies in which it holds at least 75 % of the capital or voting rights or any eligible person under the legal provisions applicable on the date of grant of Founders’ Warrants (hereinafter the “Beneficiaries of Founders’ Warrants”),
decides, in accordance with the provisions of paragraph III of Article 163 bis G of the French General Tax Code, to delegate the decision to issue and allocate Founders’ Warrants and to establish the list of beneficiaries of Founders’ Warrants and the percentage of Founders’ Warrants allocated to each Beneficiary of Founders’ Warrants thus appointed to the Board of Directors,
consequently, authorizes the Board of Directors, under the foregoing terms, to issue and allocate Founders’ Warrants, in one or more instalments for all or part of Founders’ Warrants Beneficiaries,
decides to grant a delegation to the Board of Directors to set, for each Beneficiary of Founders’ Warrants, the terms of Founders’ Warrants, including the timetable for exercising Founders’ Warrants, it being specified that these Founder Warrants shall be exercised no later than ten (10) years from their issuance and that the Founders’ Warrants which has not been exercised at the end of this ten (10) year period shall automatically lapse,
decides that this authorization shall end and that the Founders’ Warrants which would not have yet been allocated by the Board of Directors shall automatically lapse on the earliest of the following dates: (i) on expiry of a period of 18 months from the General Meeting, or (ii) the date, on which the conditions provided for in Article 163 bis G of the French General Tax Code ceased to be met, it being specified that this delegation terminates any previous delegation having the same purpose,
decides that each Founders’ Warrant will allow the subscription, under the conditions of Article 163 bis G III of the French General Tax Code and under the conditions defined hereinafter, of one ordinary share with a nominal value of one euro (€1) at an exercise price, determined by the Board of Directors on the date of allocation of Founders’ Warrants, it being specified that this price must be at least equal:
124
| • | in the event of the completion of one or more capital increases in the six months preceding the implementation of this delegation by the Board of Directors, at the subscription price of the ordinary share used during the most recent of the said capital increases assessed on the date of allocation of each Founders’ Warrants, less, where applicable, a discount corresponding to the loss of economic value of the ordinary share since this issuance; |
| • | for any allocation that takes place outside the assumptions referred to in the two points above, at the weighted average price over the last 20 trading days preceding the date of allocation of the said Founders’ Warrants by the Board of Directors; |
it being specified that, in order to set the subscription price of an ordinary share upon the exercise of a Founders’ Warrant, the Chairman will not take into account the capital increases resulting from the exercise of subscription warrants, for founders’ shares or stock options, or from the allocation of free shares,
decides that the ordinary shares thus subscribed for will have to be fully paid up at the time of their subscription, by payment in cash, including by offsetting against liquid and payable claims,
decides that the new shares delivered to each Beneficiary of Founders’ Warrants upon the exercise of their Founders’ Warrants will be subject to all the statutory provisions and will carry full rights from the first day of the financial year in which they are issued,
decides that, in accordance with Article 163 bis G-II of the French General Tax Code, Founders’ Warrants will be non-transferable, that they will be issued in registered form and will be registered in an account,
decides on the issuance of an ordinary shares, to which the exercise of the Founders’ Warrants issued will entitle,
specifies that pursuant to the provisions of Articles L. 228-91 and L. 225-132 of the French Commercial Code, this decision entails for the holders of Founders’ Warrants a waiver of their preferential subscription right of ordinary shares, to which the Founders’ Warrants entitle.
decides, as provided for in Article L. 228-98 of the French Commercial Code, that the Company is authorized, without having to seek the authorization of the holders of the Founders’ Warrants, to change its form and its corporate purpose,
decides that pursuant to the provisions of Article L. 228-98 of the French Commercial Code, the Company is authorized to modify the rules for the distribution of its profits, amortise its capital and create preference shares resulting in such modification or amortisation, subject to taking the necessary measures to maintain the rights of securities holders giving access to the share capital under the conditions defined in Article L. 228-99 of the French Commercial Code,
authorises the Company to impose on Founders’ Warrant holders the redemption or liquidation of their rights as provided for in Article L. 208-102 of the French Commercial Code,
decides to grant all powers to the Board of Directors to implement this delegation, with the option of sub-delegation within the limits and under the conditions permitted by law as specified above, including to:
| • | issue and allocate the Founders’ Warrants and to set the conditions of exercise and the final terms of the Founders’ Warrants, including the exercise timetable, in accordance with the provisions of this resolution and within the limits set in this resolution; |
125
| • | note the number of ordinary shares issued as a result of the exercise of the Founders’ Warrants, carry out the formalities resulting from the corresponding capital increases and amend the bylaws accordingly; |
| • | take steps to ensure the protection of the Founders’ Warrant holders in the event of a financial transaction involving the Company, in accordance with the legal and regulatory provisions in force; |
| • | in general, take any measure and carry out any formality useful to this issuance. |
resolves that this delegation shall be valid for a period of eighteen (18) months, starting from the date of this general meeting;
resolves that this delegation of authority shall deprive of its effects any previous delegation with the same subject matter, where applicable, for the unused part of the latter delegation.
TWELFTH RESOLUTION
Authorization granted to the Board of Directors to proceed with the free allocation of shares, existing or to be issued (the “Free Shares”), with cancellation of the preferential subscription right of shareholders in favour of a category of persons
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings;
having reviewed the reports (i) of the Board of Directors; and (ii) of the Statutory Auditor;
pursuant to Articles L. 225-197-1 et seq. and L. 22-10-59 et seq. of the French Commercial Code,
authorises the Board of Directors to allocate, in one or more times, free shares, existing or to be issued by the Company, to the Company’s employees, or certain categories of employees and/or corporate officers of the Company who meet the conditions set out in Article L. 225-197-1, II of the French Commercial Code, and to employees of the companies or economic interest groups in which the Company holds, directly or indirectly, at least ten percent (10%) of the capital or voting rights as of the date of allocation of the shares concerned,
decides to set the maximum nominal amount of capital increases that may be carried out immediately or at term under this authorization at 10% of the total number of shares comprising the Company’s capital, at the date of their allocation by the Board of Directors; it is specified that this number will be charged against the global ceiling described in the 13th resolution of this general meeting,
decides that the allocation of the shares to their beneficiaries will be final, subject to meeting the conditions or criteria that may be set by the Board of Directors, after a period of at least one (1) year (the “Vesting Period”) and that the beneficiaries of these shares must, if necessary, keep them for a period set by the Board of Directors (the “Lock-up Period”) which, together with the Vesting Period, may not be less than two (2) years,
decides, by way of derogation from the foregoing, that the shares will be finally allocated before the end of the Vesting Period in the event of disability of the beneficiary corresponding falling within the second and third categories provided for in Article L. 341-4 of the French Social Security Code,
decides that the shares allocated will be freely transferable in the event of a request for allocation made by the heirs of a deceased beneficiary or in the event of disability of the beneficiary falling within the aforementioned categories of the French Social Security Code,
126
decides that the length of the Vesting Period and of the Lock-up Period will be set by the Board of Directors within the above-mentioned limits,
takes note that, in accordance with the provisions of Article L. 25-197-1 of the French Commercial Code, when the allocation relates to the shares to be issued, this authorization automatically entails, in favour of the beneficiaries of the free shares, a waiver by the shareholders of their preferential subscription rights to the new shares issued, with the corresponding capital increase being finally completed by the sole fact of the final allocation of the shares to the beneficiaries,
takes note that this resolution entails, as necessary, the waiver by the shareholders, in favour of the beneficiaries of free shares, of the part of the reserves, profits or premiums which, if need be, will be used in the event of the issuance of new shares at the end of the Vesting Period, for the completion thereof all powers are delegated to the Board of Directors to carry out such issuance,
delegates all powers to the Board of Directors to:
| • | ascertain that sufficient reserves are existing and proceed at the time of each allocation to the transfer to an unavailable reserve account of the sums necessary for the release of the new shares to be allocated, |
| • | set the identity of the Beneficiaries and the number of free shares that may be allocated to each; |
| • | set the conditions and, as the case may be, the criteria for allocating these shares; where appropriate, |
| • | decide, in due time, the capital increase or share capital increases resulting from the issuance of any new free shares, |
| • | proceed to acquire the shares as may be necessary for the delivery of any existing shares allocated as free shares; |
| • | take all necessary measures to ensure that the beneficiaries satisfy their obligation to retain such free shares; |
| • | and, in general, to do within the framework of the legislation in force all that is required by the implementation of this authorization; |
resolves that this authorization shall be valid for a period of thirty-eight (38) months starting from this general meeting;
resolves that, as from its implementation, this authorization shall deprive of its effects any previous authorization with the same subject matter.
THIRTEENTH RESOLUTION
Setting of overall limits of amount of issuances carried out pursuant to authorizations to grant Options and Free Shares and to delegations to issue Warrants and Founders’ Warrants
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings;
Having reviewed the reports (i) of the Board of Directors; and (ii) of the Statutory Auditor;
resolves that the sum of the shares likely to be issued or allocated (i) upon exercise of the Options that would be granted pursuant to the 9th resolution above; (ii) the shares likely to be issued upon exercise of the warrants that would be allocated pursuant to the 10th resolution above; (iii) the shares likely to be issued upon exercise of the founder’s warrants that would be allocated pursuant to the 11th resolution
127
above and (iv) the shares that may be issued pursuant to the shares allocated free of charge pursuant to the 12th resolution above may not exceed 15% of the share capital recorded on the date of the decision of allocation or issuance, it being specified that this limit may be increased by the additional amount of the shares to be issued to preserve, pursuant to legal provisions and, as the case may be, under applicable contractual terms, the rights of holders of securities or other rights giving access to the shares.
FOURTEENTH RESOLUTION
Delegation to the Board of Directors to carry out a share capital increase by issuing shares or securities giving access to the share capital, reserved for members of a company savings plan with cancellation of the preferential subscription right in favour thereof
The General Meeting,
ruling under the conditions of quorum and majority for extraordinary general meetings;
having reviewed the reports (i) of the Board of Directors; and (ii) of the Statutory Auditor;
taking note of the provisions of Articles L. 3332-18 to L. 3332-24 of the French Labour Code, and ruling pursuant to the provisions of Articles L. 225-129-6 and L. 225-138-1 of the French Commercial Code,
delegates to the Board of Directors its authority, with the option of sub-delegation to the Chief Executive Officer and/or to the Deputy Chief Executive Officer, to decide on the increase of the share capital, from time to time, at the time and pursuant to the terms and conditions it determines, for a maximum nominal amount of 3% of the share capital at the date of issuance, through the issuance of ordinary shares or financial securities giving access to the Company’s share capital, reserved for members of a company savings plan (or any other plan for members of which Articles L. 3332-1 et seq. of the French Labour Code or any similar law or regulation that would allow a capital increase to be reserved under equivalent conditions), implemented or to be implemented within the Company, it being specified that this maximum nominal amount above shall be increased by the securities issued in order to preserve the rights of holders of securities giving access to the share capital pursuant to the provisions of the French Commercial Code;
resolves that the subscription price of the shares shall be set pursuant to the provisions of Article L. 3332-20 of the French Labour Code;
resolves that this delegation entails the cancellation of the preferential subscription right of the shareholders to the new shares or securities to be issued in favour of the aforementioned beneficiaries, in the event of completion of the capital increase provided for in the paragraph above;
resolves that the Board of Directors may provide for the free allocation of shares or financial securities giving access to the Company’s share capital; under the terms provided for in Article L. 3332-21 of the French Labour Code;
resolves that each capital increase shall only be carried out up to the amount of the ordinary shares actually subscribed by the aforementioned beneficiaries;
resolves that the characteristics of the issuance of financial securities giving access to the company’s capital shall be set by the Board of Directors under the conditions provided for by regulations;
128
grants all powers to the Board of Directors to implement this delegation and, in particular to:
| • | decide and set the terms and conditions for the issuance and allocation of shares or financial securities giving access to the share capital, pursuant to this delegation; in particular to set the subscription price pursuant to the rules defined above, the opening and closing dates of subscriptions, the dates of enjoyment (even if retroactive), the deadlines for paying up the shares and, where applicable, the financial securities giving access to the capital, all within the legal limits; |
| • | record the completion of the share capital increase(s) up to the amount of the shares or securities that will be effectively subscribed and make the related amendments to the bylaws; |
| • | carry out all operations and formalities, directly or by proxy; |
| • | and in general, do everything useful and necessary for the final completion of the share capital increase or share capital increases; |
resolves that this delegation shall be valid for a period of eighteen (18) months, starting from the date of this general meeting,
resolves that this delegation of authority shall deprive of its effects any previous delegation with the same subject matter, where applicable, for the unused part of this latter delegation.
FIFTEENTH RESOLUTION
Powers for formalities
The General Meeting,
ruling under the conditions of quorum and majority for ordinary general meetings,
grants full powers to the bearer of an original, copy or excerpt of these minutes to carry out all filing, publication and other formalities as necessary.
129
ANNEX 4. EXTRACTS FROM THE RESOLUTIONS OF THE BOARDS OF DIRECTORS OF ERYTECH AND PHERECYDES AUTHORIZING THE MERGER PROJECT
[…]
I - Approval and authorization to sign the Merger Agreement
The Chairman presented the main terms and conditions of the Merger Agreement that the Company intends to enter into with Pherecydes, the draft of which is attached as Annex 2:
| • | Universal transfer of assets and liabilities: Pherecydes, as the absorbed company, will contribute all the assets and liabilities of its assets to the Company, as the absorbing company. The completion of the Merger will result in the dissolution without liquidation of Pherecydes and the transfer of all its assets and liabilities to the Company. As a result of the Merger, the Company will be subrogated in all the rights and obligations of Pherecydes; |
| • | Consultation of the employees: the social and economic committee of the Company (the “CSE”) has been informed and consulted and has issued, on March 20, 2023, a favorable opinion on the Merger; |
Reference financial statements: the terms and conditions of the Merger are established on the basis of the financial statements of the Company and Pherecydes for the fiscal year ending December 31, 2022;
| • | Valuation of the assets contributed by Pherecydes: as this is an “upfront” transaction involving entities under separate control, within the meaning of ANC Regulation No. 2014-03 relating to the general chart of accounts of June 5, 2014, as amended by ANC Regulation No. 2022-01 of March 11, 2022, the assets contributed by Pherecydes, and the liabilities assumed by the Company in the context of the Merger are valued at their real value. On this basis, the real value of the assets contributed by Pherecydes amounts to 16,537,386 euros; |
| • | Merger Auditor: the terms and conditions of the Merger, the relative values attributed to the shares of the Company and Pherecydes and the Exchange Ratio have been submitted to the assessment of the firm Finexsi, represented by Mr. Christophe Lambert, acting as merger auditor as appointed by order of the president of the commercial court of Lyon dated February 28, 2023, in accordance with the terms of Articles L. 225-147, L. 227-1, L. 236-10 et seq, R. 210-19, R. 225-7 et seq. and R. 236-6 of the French Commercial Code, and the report of the merger auditor will be filed with the competent commercial court in accordance with the aforementioned articles of the French Commercial Code (the “ Merger Auditor “); |
| • | Exchange Ratio: in order to determine the remuneration of the Pherecydes Merger, a valuation of the real value of Pherecydes and the Company, as well as of their shares, has been performed. |
The methods used to value the Pherecydes Merger Consideration and the reasons for the choice of the Exchange Ratio are explained by the Chairman.
130
On this basis, the exchange ratio retained in the context of the Merger is four (4) ordinary shares of Pherecydes for fifteen (15) ordinary shares of the Company (the “Exchange Ratio”);
| • | Remuneration of the net assets contributed by Pherecydes: as remuneration and representation of the net assets contributed by Pherecydes, the shareholders of Pherecydes (with the exception of Pherecydes with respect to the treasury shares and the Company with respect to the shares held as a result of the Contributions in Kind) will be allocated 26,575,893 new shares of ten euro cents (€0,10) par value each, fully paid up, to be created by the Company, according to the above-mentioned Exchange Ratio (the “New Shares”); |
| • | Capital increase of the Company and merger premium: in consideration of the contributions made by Pherecydes to the Company, other than those subject to a merger-waiver, the Company will increase its share capital by an amount of 2,657,589.30 euros, through the creation of 26,575,893 New Shares with a par value of ten euro cents (€0,10) each, with a balancing payment in cash of a total amount of forty-two euro cents (€0.42), it being specified that the final number of New Shares to be issued and consequently the nominal amount of the resulting capital increase will be adjusted by operation of law according to the exact number of Pherecydes shares to be remunerated pursuant to the Merger (the “Capital Increase”). |
Without prejudice to the provisions relating to fractional shares, the Company will not pay any compensation for any balancing payment and the shareholders of Pherecydes expressly waive the payment of any balancing payment.
The New Shares will be entitled to dividends as from the Completion Date (as defined below) and will be fully assimilated to the existing ordinary shares of the Company. They will have the same rights and will bear the same charges.
The New Shares will be negotiable as soon as the Capital Increase is completed and will immediately be the subject of an application for admission to trading on compartment C of the regulated market of Euronext Paris. It is specified that the New Shares will neither be registered with the Securities Exchange Commission (SEC) nor be the subject of an application for admission to trading on Nasdaq.
In addition, the Merger will generate a merger premium amounting to 12,099,841.12 euros;
| • | Fractional shares: the fractional shares will not be tradable or transferable and, as a consequence, if the number of shares of the Company to which a shareholder of Pherecydes is entitled does not correspond to a whole number of shares of the Company, such shareholder will receive the number of shares of the Company immediately below, plus, for the entirety of the remaining number of shares, a cash payment corresponding to the price at which the shares of the Company corresponding to the fractional shares will have been sold, paid by the financial intermediaries; |
| • | Securities giving access to the share capital and free shares: with respect to the existence and circulation of securities giving access to the share capital of Pherecydes, the Merger will result in the assumption of the commitments of Pherecydes with respect to the allocation of free ordinary shares by the latter (the “AGAs”) and the issuance of warrants to subscribe for founder’s share warrant issued by the latter prior to the completion of the Merger (the “BSPCE”). As from the Completion Date, (i) the Company will be substituted by operation of law for Pherecydes in its obligations towards the beneficiaries of the AGAs and the holders of the BSPCEs and (ii) the Exchange Ratio will be applied according to the following |
131
| formalities the number of ordinary shares of Pherecydes to which each beneficiary would be entitled in the case of the same plan of allocation will correspond to the number of ordinary shares of the Company to which he/she would have been entitled under this plan multiplied by the Exchange Ratio, the number thus obtained at the time of the definitive acquisition of the AGAs or the exercise of the BSPCEs being rounded down to the nearest whole number, without payment of a cash balance; |
| • | Opposition of the debtors: the debtors of Pherecydes and of the Company, whose claim will be prior to the publication of the Merger Agreement, will be able to object within a period of thirty (30) days as from the publication of the Merger Agreement; |
| • | Conditions precedent: the Merger and the Capital Increase will only become final subject to, and solely as a result of, the lifting of the following conditions precedent (the “Merger Conditions precedent”): |
| • | the delivery by the Merger Auditor of (i) a report on the value of the contributions and (ii) a report on the terms and conditions of the Merger confirming the fairness of the Exchange Ratio; |
| • | the approval by the general shareholders’ meeting of Pherecydes of the Merger and the resulting dissolution of Pherecydes; |
| • | the approval by the Company’s shareholders’ meeting of the Merger and the related Capital Increase, of the resolutions relating to the appointment of the directors designated by Pherecydes and of the amendment of the Company’s by-laws relating to the removal of the casting vote of the chairman of the board of directors. |
| • | Completion date of the Merger: the Merger and the related Capital Increase will be definitively completed on the date of completion of the last of the Conditions Precedent to the Merger, i.e. on the date of the last of the general meetings of the shareholders of the Company and of Pherecydes, on June 23, 2023, at 11:59 p.m. (the “Completion Date”). The Chairman reminds that in the event that the Merger Conditions are not satisfied by midnight on July 31, 2023, at the latest, the Merger Agreement will be deemed null and void. |
| • | Effective date of the Merger: from an accounting and tax point of view, the Merger will take effect retroactively as of January 1, 2023. The profit or loss generated by Pherecydes since January 1, 2023 will be included in the taxable income of the Company. |
| • | Representations and warranties: the Merger Agreement includes customary representations and warranties from the Company and Pherecydes; |
| • | Tax regime: the Merger is subject to the preferential tax regime set forth in Article 210 A of the French General Tax Code. Consequently, the unrealized capital gains on all the contributed assets, as well as the provisions (other than those becoming irrelevant), will not be subject to corporate income tax at the level of Pherecydes pursuant to the provisions of Article 210 A of the French General Tax Code. |
The President then opened discussion with the members of the Board of Directors. All explanations are given in response to the questions asked.
132
After having deliberated thereon, the Board of Directors, having reviewed the terms and conditions of the Merger and the draft Merger Agreement, unanimously by the members of the Board of Directors having taken part in the vote :
| • | approved the principle of the Merger and all the terms and conditions set forth in the Merger Agreement, as well as the related Capital Increase ; |
| • | considered that the Merger and the conclusion of the Merger Agreement are in the interest of the Company; |
| • | authorized the execution by the Company of the Merger Agreement; and |
| • | authorized the CEO of the Company, with the right of delegation, to adapt, finalize, conclude, sign and deliver the Merger Agreement and more generally all agreements and documentation necessary for the completion and implementation of the Merger, to make all payments and payments related thereto, and more generally to take all measures, sign all deeds, make all declarations and carry out all formalities that are necessary or useful for the implementation of the present decision and the completion of the Merger transactions. |
The Board of Directors further grants to Gil Beyen, all powers to prepare and sign the declaration of compliance provided for in Article L. 236-6 of the French Commercial Code.
[…]
133
English translation of the French original for information purposes only
PHERECYDES PHARMA
French Société anonyme with a Board of Directors, with a share capital of €7,939,179
Registered office: Nantes Biotech, 22, Boulevard Benoni Goullin - 44200 Nantes
(France) 493 252 266 RCS Nantes
(the “Company”)
EXTRACT FROM THE MINUTES OF THE DECISIONS OF THE BOARD OF DIRECTORS
DATED MAY 5, 2023
The year two thousand and twenty-three,
On May 5,
At 10:00 a.m,
The members of the Company’s Board of Directors (the “Board of Directors” or the “Board”) met in accordance with the provisions of Article L. 225-37 of the French Commercial Code, at the invitation of the Chairman of the Board of Directors.
[...]
Mr. Didier Hoch chairs the meeting in his capacity as Chairman of the Board of Directors.
Mr. Thibaut du Fayet acts as secretary of the meeting.
The President notes that at least half of the members are present. The conditions of quorum being satisfied, the Board can validly deliberate.
[...]
The following documents have been previously made available to the members of the Board of Directors (the “Documents”):
[...]
| • | The draft merger agreement between (i) Erytech Pharma, as the Absorbing Company, as defined below, and (ii) the Company, as the Absorbed Company, as defined below (the “Draft Merger Agreement”); |
| • | [...] |
The President reminds the Board of Directors that the meeting had been called to deliberate on the following agenda:
Agenda:
[...]
3. Review and Approval of the Draft Merger Agreement;
[...]
134
The Chairman reminds the directors that, pursuant to a memorandum of understanding (the “MoU”) entered into on February 15, 2023 between Erytech Pharma, a French société anonyme (limited liability company), whose registered office is located at 60, avenue Rockefeller, 69008 Lyon (France), and registered with the Lyon Trade and Companies Registry under number 479 560 013 (“Erytech”, or the “Absorbing Company”) and the Company (“Pherecydes” or the “Absorbed Company”), Erytech and the Company have agreed on the terms of a proposed merger of Pherecydes into Erytech, with an exchange ratio pursuant to which Pherecydes shareholders would receive 15 Erytech shares in exchange for 4 Pherecydes shares (the “Proposed Merger”).
In accordance with the terms of the MoU, Erytech and Pherecydes have further discussed the terms of the Proposed Merger, Erytech’s business prospects, governance and corporate name of Erytech following the completion of the Proposed Merger, and have decided to confirm their commitments by entering into a merger agreement (the “Draft Merger Agreement”).
[...]
REVIEW AND APPROVAL OF THE PROPOSED MERGER
The Chairman presents the main terms and conditions of the Draft Merger Agreement, namely:
| • | The Absorbed Company would contribute all of its assets, and the Absorbing Company would take over all of its liabilities; at the completion of the Proposed Merger, the assets of Pherecydes would be transferred on a universal basis to Erytech, and Pherecydes would be dissolved without liquidation; |
| • | The Draft Merger Agreement is based on the financial statements of Pherecydes and Erytech for the year ended December 31, 2022; |
| • | As this is an “upright” transaction involving entities under separate control, within the meaning of ANC Regulation No. 2014-03 relating to the French plan comptable géneral of June 5, 2014, as amended by ANC Regulation No. 2022-01 of March 11, 2022, the assets contributed by the Company and the liabilities assumed by Erytech in the context of the Proposed Merger are valued at their fair market value; |
| • | In order to determine the consideration for the Company’s merger, the fair market value of the Company and Erytech, as well as their shares, was assessed. The methods used to value the Company’s merger consideration and the reasons for the choice of the exchange ratio were explained by the Chairman, who stated that on this basis, the exchange ratio retained in the framework of the Proposed Merger is four (4) ordinary shares of Pherecydes for fifteen (15) ordinary shares of Erytech; |
| • | As consideration and representation of the net assets contributed by the Company, the shareholders of the Company (except for the Company with respect to the treasury shares and Erytech with respect to the shares held at the end of the Contribution in Kind and at the Completion Date as defined in the Merger Agreement) will be granted 26,575,893 new shares of €0.10 (ten cents) each, fully paid-up, to be issued by the Absorbing Company, according to the above-mentioned Exchange Ratio (the “New Shares”), it being specified that the final number of new shares to be issued and, accordingly, the nominal amount of the resulting share capital increase will be adjusted by operation of law according to the exact number of Pherecydes Shares to be remunerated under the Merger; |
135
| • | The New Shares will be fully paid-up and assimilated to the existing ordinary shares. They would have the same rights and would be subject to all the provisions of Erytech’s by-laws. They would also be admitted to trading on the regulated market Euronext Paris, and will be immediately assimilated to the existing ordinary shares of Erytech; it is specified that the New Shares will neither be registered with the Securities Exchange Commission (SEC) nor be the subject of an admission to trading on Nasdaq; |
| • | The fractional rights (“rompus”) would not be negotiable or transferable and, consequently, when the number of Erytech shares to which a Pherecydes shareholder would be entitled would not correspond to a full number of shares of the Absorbing Company, the shareholder would receive the number of shares of the Absorbing Company immediately below, plus, for the entirety of the balance, a cash balance (“soulte”) corresponding to the price at which the shares of the Absorbing Company corresponding to the fractional shares will have been sold, paid by the financial intermediaries referred to in article 14.4 of the Draft Merger Agreement; |
| • | In addition, with respect to the existence and circulation of securities giving access to the share capital of Pherecydes, the Proposed Merger would result in the assumption, in accordance with (i) the provisions of Articles L. 225-197-1 and L. 228-98 to L. 228-106 of the French Commercial Code and (ii) the Draft Merger Agreement, of the commitments of the Company with respect to the free granting of ordinary shares by the latter (the “AGAs”) and the issuance of founders’ warrants (the “BSPCEs” or “Founders’ Warrants”) issued by the latter prior to the completion of the Proposed Merger; |
| • | As from the Completion Date (as this term is defined in the Draft Merger Agreement), (i) Erytech would be substituted by operation of law for the Company’s obligations towards the beneficiaries of the AGAs and the holders of the BSPCEs and (ii) the exchange ratio set forth in Article 14.1 of the Draft Merger Agreement would apply according to the following formalities: the number of ordinary shares of Pherecydes to which each beneficiary would be entitled in the case of the same plan will correspond to the number of ordinary shares of Erytech to which he would have been entitled pursuant to such plan multiplied by the merger ratio applicable to the shareholders referred to in Article 14.1 of the Draft Merger Agreement, the number thus obtained at the time of the definitive acquisition of the AGAs or the exercise of the BSPCEs being rounded down to the nearest whole number, without payment of a cash balance. |
| • | The Proposed Merger and the related Erytech share capital increase will only become final subject to, and solely as a result of, the completion of the following conditions precedent (the “Conditions Precedent”): |
| • | The delivery by Finexsi (the independent merger appraiser (“commissaire à la fusion”)) of its report on the value of the contributions and on the terms and conditions of the Proposed Merger, confirming the fairness of the envisaged exchange ratio; |
| • | The approval by the general shareholders’ meeting of the Company of the Merger and the resulting dissolution of the Company; |
| • | The approval by the general shareholders’ meeting of the Absorbing Company of the Merger as well as the related capital increase, and of the resolutions relating to the appointment of directors chosen by Pherecydes and to certain amendments to the Erytech’s by-laws of in accordance with the provisions of the Draft Merger Agreement. |
The President reminds that the creditors of Pherecydes and the Absorbing Company whose claims are prior to the publication of the Proposed Merger Agreement may file an objection within thirty (30) days from the publication of the Draft Merger Agreement;
136
The Completion Date of the Merger and the automatic dissolution of the Company would be set on the day of the fulfillment of the last of the conditions precedent set forth in Article 16 of the Draft Merger Agreement, corresponding to the day of the last of the general meetings of the shareholders of Erytech and Pherecydes, (at this stage, on June 23, 2023), at 11:59 p.m. , it being specified that the Merger would retroactively take effect, from a tax and accounting point of view, as of January 1st, 2023. The Chairman reminds that if the conditions precedent are not fulfilled on July 31, 2023 at midnight at the latest, the Draft Merger Agreement will be considered as null and void.
In order to facilitate the Merger, it is proposed to the Board to decide that as from the date hereof and until the Completion Date of the Merger, the share repurchase program will no longer be used and to not proceed to any cancellation of the treasury shares.
The President then gives the floor to the directors. All explanations are given in response to the questions from the directors.
After having deliberated, the Board, unanimously of the members present, approves and authorizes the Draft Merger Agreement to be entered into between Erytech, as the Absorbing Company, and the Company, as the Absorbed Company, as presented in detail.
The Board of Directors also decides, in order to facilitate the Merger, that as from the date hereof and until the Completion Date, the share repurchase program of the Company shall no longer be used and that the treasury shares shall not be cancelled.
The Board of Directors grants the Chief Executive Officer of the Company, with the option of sub delegation, the following powers, which are not exhaustive:
| • | To execute the Draft Merger Agreement, and to make, as the case may be, if it should prove necessary and that it should deem useful, any amendment, clarification or addition thereto; |
| • | To execute all additional, reiterative or amending documents, elect domicile, carry out all formalities provided for by law and generally do all that is necessary or useful for the completion of the Proposed Merger; and |
| • | To suspend the exercise of the rights of the holders of securities giving access to the share capital of the Company, at the latest on the date of the convening notice of the shareholders’ meeting, in order to facilitate the Merger and to freeze the share capital of the Company, for a maximum three month period. |
The Board of Directors authorizes Mrs. Leïla Nicolas and Mr. Didier Hoch, directors, each of whom may act separately, to sign on behalf of the Company the declaration of regularity and conformity (“declaration de régularité et de conformité”) provided for in Article L. 236-6, paragraph 3, of the French Commercial Code.
[...]
137
POWERS TO CARRY OUT FORMALITIES
The Board of Directors gives full powers to the bearer of the original, a copy or an extract of these minutes to carry out with the clerk of the Commercial Court all filings and formalities of legal and other publicity that may be required.
[...]
***
There being no further business, the President adjourns the meeting at 1:00 p.m.
Of all the above, the present minutes have been drawn up and signed by the President and one director.
Extract certified as true. The Chairman of the Board
138
ANNEX 5. CONCORDANCE TABLE
This concordance table identifies the main information provided for in Annex 1 of the Delegated Regulation (EU) 2021/528 of December 16, 2020 (the “Delegated Regulation”) included in the documents incorporated by reference for the purposes of the Exemption Document.
| Annex 1 to Delegated Regulation
(EU) |
Sections of the |
Documents incorporated by |
Chapters/Sections of |
Pages of the document incorporated by reference | ||||||
| Item |
||||||||||
| 2.2 |
Business overview | |||||||||
| 2.2.1 |
Principal activities, including the main categories of products sold and/or services performed in the last financial year. | Section 2.1.2.1 (Erytech) | Erytech 2022 Universal Registration Document | Sections 1.3, 1.4, 1.5 and 1.6 | Pages 10 to 22
| |||||
| Section 2.2.2.1 (Pherecydes) | Pherecydes 2022 Annual Financial Report | Chapter 1 | Pages 5 and 6 | |||||||
| 2.2.2 |
Any significant changes having an impact on the operations and principal activities since the end of the period covered by the latest published audited financial statements. | Section 2.1.2.2 (Erytech) | Erytech 2022 Universal Registration Document | Section 1.1 | Pages 7 to 9
| |||||
| Section 2.2.2.2 (Pherecydes) |
Pherecydes 2022 Annual Financial Report |
Section 1.7 | Pages 20 and 21 | |||||||
139
| 2.2.3 |
A brief description of the principal markets, including a breakdown of total revenues by operating segment and geographic market for the last financial year.
|
Section 2.1.2.3 (Erytech) | Erytech 2022 Universal Registration Document | Section 1.8 | Page 25 | |||||
| 2.4 |
Corporate Governance | |||||||||
| 2.4.1 |
Names, business addresses and functions within the issuer or, depending on the type of transaction, the offeree company, the company being acquired or the company being divided, of the members of the administrative, management or supervisory bodies and, in case of a limited partnership with a share capital, of partners with unlimited liability.
|
Section 2.1.4.1 (Erytech) | Erytech 2022 Universal Registration Document | Section 3.1.1.2 | Pages 96 to 114 | |||||
| Section 2.2.4.1 (Pherecydes) | Pherecydes 2022 Annual Financial Report | Chapter 1 | Pages 43 to 54
| |||||||
| 2.4.2 |
Identity of major shareholders. | Section 2.1.4.2 (Erytech) | Erytech 2022 Universal Registration Document | Section 4.1 | Pages 167 to 168
| |||||
| Section 2.2.4.2 (Pherecydes) | Pherecydes 2022 Annual Financial Report | Section 6.1 | Page 28
| |||||||
| 2.4.3 |
Number of employees. | Section 2.1.4.3 (Erytech) | Erytech 2022 Universal Registration Document | Section 1.13.1.1 | Page 33
| |||||
| 2.5 |
Financial information | |||||||||
| 2.5.1 |
Financial statements | Section 2.1.5.1 (Erytech) | Erytech 2022 Universal Registration Document | Sections 5.3.1, 5.3.2, and 5.3.3, 5.3.4 | Pages 201 to 301
| |||||
140
| Section 2.2.5.1 (Pherecydes) | Pherecydes 2022 Annual Financial Report | Annex to the Pherecydes 2022 Annual Financial Report. | Pages 67 to 89 of the Pherecydes 2022 Annual Financial Report | |||||||
| 2.5.3 |
A description of any significant change in the financial position which has occurred since the end of the last financial period for which either audited financial statements or interim financial information have been published, or where no such significant change has occurred, a statement to that effect. | Section 2.1.5.3 (Erytech)
|
Erytech 2022 Universal Registration Document
|
Section 5.3.6
|
Page 302 | |||||
| Section 2.2.5.3 | Pherecydes 2022 Annual Financial Report | Section 3.2 of the notes to the financial statements for the year ended December 31, 2022. | Page 77
| |||||||
| 2.5.4 |
Management report | Section 2.1.5.4 | Erytech 2022 Universal Registration Document | Please refer to the index on page 3 of the cross-reference tables in the Erytech 2022 Universal Registration Document. | Page 3 of the concordance tables | |||||
141
| Section 2.2.5.4 |
Pherecydes 2022 Annual Financial Report |
First part of the Pherecydes 2022 Annual Financial Report | Pages 1 to 42 | |||||||
| 3.3 |
[Risk Factors] | |||||||||
| 3.3 |
A description, in a limited number of categories, of the material risks that are specific to the transaction, in a section headed “Risk factors relating to the transaction”.
In each category, the most material risk factors in the assessment of the issuer, taking into account the negative impact on the issuer and the probability of their occurrence, shall be mentioned first.
The risk factors shall be corroborated by the content of the exemption document. |
Section 3.3 | Erytech 2022 Universal Registration Document | Chapter 2 | Pages 66 to 93 | |||||
142
Exhibit 99.2
PHERECYDES PHARMA
Limited company (société anonyme) with a Board of Directors and capital of €7,939,179
Registered office: 22, Boulevard Benoni Goullin – 44200 Nantes, France
493 252 266 Nantes Trade and Companies Register
2022 ANNUAL FINANCIAL REPORT
1
STATEMENT BY THE PERSON RESPONSIBLE FOR THE 2022 ANNUAL FINANCIAL REPORT
(Article 222-3-4° of the AMF General Regulation)
“I certify that the information contained in this financial report is, to the best of my knowledge, in accordance with the facts and contains no omission likely to affect its scope.”
“I certify that, to the best of my knowledge, the accounts have been prepared in accordance with the applicable set of accounting standards and give a true and fair view of the assets, liabilities, financial position and profit or loss of the Company, and that the accompanying management report presents a true and fair view of the development of the Company’s business, profit or loss and financial position, together with a description of the principal risks and uncertainties that it faces.”
Chief Executive Officer
Thibaut du Fayet
2
PHERECYDES PHARMA
Limited company (société anonyme) with a Board of Directors and capital of €7,939,179
Registered office: 22, Boulevard Benoni Goullin – 44200 Nantes, France
493 252 266 Nantes Trade and Companies Register
MANAGEMENT REPORT
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2022
Dear Shareholders,
We have called this Annual General Meeting of Shareholders in accordance with the articles of association and the French law on commercial companies, to report to you on the activities of Pherecydes Pharma (hereinafter the “Company”) during the fiscal year ending December 31, 2022, the results of these activities and future outlooks, and to submit the annual financial statements for said fiscal year for your approval.
The notices of meeting required by law will be regularly sent to you, and all the documentation provided for by applicable regulations will be made available to you within the time frame established.
TABLE OF CONTENTS
| CHAPTER 1 - |
Activity of the Company and development of its business during the fiscal year ended December 31, 2022 | 5 | ||||
| 1.1 |
General presentation of the Company’s activity | 5 | ||||
| 1.2 |
Status fo the activity and analysis of the development of the business during the year 2022 | 6 | ||||
| 1.3 |
Results of the activity, progress made and difficulties encountered | 15 | ||||
| 1.3.1 |
Economic and financial results | 15 | ||||
| 1.3.2 |
Results for the fiscal year ended December 31, 2022 | 16 | ||||
| 1.4 |
Research and development activities | 16 | ||||
| 1.5 |
Polluting or risky activities | 16 | ||||
| 1.6 |
Main risks and uncertainties facing the company and financial risk management | 17 | ||||
| 1.6.1 |
Liquidity risks | 17 | ||||
| 1.6.2 |
Risks related to historical and future losses | 17 | ||||
| 1.6.3 |
Risks of impairment of intangible assets | 18 | ||||
| 1.6.4 |
Risks relating to shares and other financial instruments | 18 | ||||
| 1.6.5 |
Dilution risks | 19 | ||||
| 1.7 |
Significant events between the balance sheet date and the reporting date | 19 | ||||
| 1.8 |
Foreseeable developments and outlook | 20 | ||||
| CHAPTER 2 - |
Subsidiaries and equity investments | 23 | ||||
| 2.1 |
Subsidiaries of the Company | 23 |
3
| 2.2 |
Acquisition of significant shareholdings in companies headquartered in France or acquisition of control of such companies | 23 | ||||
| CHAPTER 3 - |
Information on payment terms to suppliers and customers | 24 | ||||
| CHAPTER 4 - |
Total dividends distributed over the last three years | 25 | ||||
| CHAPTER 5 - |
Five year summary of results | 26 | ||||
| CHAPTER 6 - |
Breakdown of share capital and treasury shares | 27 | ||||
| 6.1 |
Breakdown of share capital during the last fiscal year | 27 | ||||
| 6.2 |
Securities giving access to the share capital | 27 | ||||
| 6.3 |
Crossing of statutory ownership thresholds | 28 | ||||
| 6.4 |
Employee shareholding | 28 | ||||
| 6.5 |
Information on treasury shares | 29 | ||||
| CHAPTER 7 - |
Transactions carried out by managers on their shares | 31 | ||||
| CHAPTER 8 - |
Compensation and benefits granted to corporate officers and shareholding | 32 | ||||
| 8.1 |
Compensation and benefits granted to corporate officers | 32 | ||||
| 8.2 |
Shareholdings of corporate officers | 34 | ||||
| CHAPTER 9 - |
Special report on stock options and free shares | 35 | ||||
| 9.1 |
Share subscription or purchase options | 35 | ||||
| 9.2 |
Free share allocations | 35 | ||||
| CHAPTER 10 - |
Appointment of the Statutory Auditors | 38 | ||||
| 10.1 |
Statutory Auditors | 38 | ||||
| 10.2 |
Statutory Auditors who resigned, were dismissed or were not reappointed during the fiscal year ended December 31, 2022 | 38 | ||||
| CHAPTER 11 - |
List of related-party agreements and current agreements | 39 | ||||
| 11.1 |
New related-party agreements entered into during fiscal year 2022 | 39 | ||||
| 11.2 |
New related-party agreements entered into since the end of fiscal year 2022 | 39 | ||||
| 11.3 |
Related-party agreements approved by the General Meeting of Shareholders, but which continue to be in effect during fiscal year 2022 | 39 | ||||
| 11.4 |
Sureties, endorsements and guarantees given by the Company to third parties | 39 | ||||
| 11.5 |
Agreements between a corporate officer or a shareholder holding more than 10% of the voting rights of the Company and a subsidiary, excluding current agreements | 39 | ||||
| CHAPTER 12 - |
Other information | 40 | ||||
| CHAPTER 13 - |
Report of the Board of Directors | 41 |
4
CHAPTER 1— ACTIVITY OF THE COMPANY AND DEVELOPMENT OF ITS BUSINESS DURING THE FISCAL YEAR ENDED DECEMBER 31, 2022
Below you will find the information required pursuant to Articles L.232-1 II and R.225-102 of the French Commercial Code.
| 1.1 | GENERAL PRESENTATION OF THE COMPANY’S ACTIVITY |
Founded in 2006, Pherecydes Pharma is a French biotechnology company that aims to develop new solutions to fight complicated and/or resistant bacterial infections. Pherecydes Pharma specializes in the treatment of bacterial infections with bacteriophages (or phages), natural viruses capable of fighting antibiotic-resistant bacteria.
As presented below, Pherecydes Pharma’s portfolio consists of assets for which the first phase II clinical trials began in spring 2022. With CPP (Comités de Protection des Personnes – institutional review board) approval obtained in February 2022, the Company announced the enrollment of the first patient in the PhagoDAIR phase II clinical trial on June 15, 2022.
An initial market launch of the bacteriophages is expected in 2026 (PhagoDAIR study, Staphylococcus aureus program).

The Staphylococcus aureus program, a bacterium considered a high global priority by the World Health Organization (WHO), is the Company’s flagship program. It includes, at this stage, three clinical trials: PhagoDAIR for which Pherecydes Pharma is the sponsor, as well as PhagOS and PhagoPied, which are financed by Hospital Clinical Research Programs (“Programmes Hospitaliers de Recherche Clinique – PHRC”) and whose sponsors will be the University Hospital Centers of Bordeaux and Nîmes. The latter two trials are expected to start in the third and second quarters of 2023 respectively.
The Company enrolled its first patient on June 15, 2022 in the PhagoDAIR phase II clinical trial and received a unanimous recommendation from the Data Safety Monitoring Board (DSMB) on November 29, 2022 to continue without modification this phase II clinical study on osteoarticular infections of prostheses caused by Staphylococcus aureus (S. aureus).
5
According to the Company, PhagoDAIR is the world’s first clinical study on precision phagotherapy in this indication.
In addition to the PhagoDAIR study, the company aims to launch, as a sponsor, another clinical study (Phase 1 PK) on infective endocarditis, for which the level of medical need is very high, as it is associated with a one-year mortality rate of around 30-40%.
| 1.2 | STATUS OF THE ACTIVITY AND ANALYSIS OF THE DEVELOPMENT OF THE BUSINESS DURING THE YEAR 2022 |
| • | A pronounced acceleration in compassionate treatment with >75 patients treated to date |
In line with the authorizations of the French National Agency for the Safety of Medicines (Agence Nationale de Sécurité du Médicament “ANSM”), more than 75 patients have treated with Pherecydes’ precision phagotherapy since early 2021.
These therapies were administered in nine hospitals, and mainly focused on S. aureus and P. aeruginosa infections. Several indications were targeted, with a high proportion of osteoarticular infections of prostheses.
Pherecydes Pharma’s phages, administered by different routes (intra-articular, intravenous, bronchoalveolar nebulization, etc.), have demonstrated excellent tolerance with no reported side effects, and an initial clinical benefit through the control of infection for nearly 75% of evaluable patients.
Pherecydes Pharma and CEA’s PhagECOLI project receives a €2 million grant from Bpifrance
On January 3, 2022, the Company and the CEA announced the selection of the PhagECOLI project submitted as part of the Emerging Infectious Diseases and New Radiological, Biological and Chemical Threats’ Call for Expressions of Interest. This project will receive a grant from Bpifrance of €2 million – 50% of its estimated cost – of which Pherecydes Pharma will receive 80% and the CEA will receive 20%.
The PhagECOLI project draws on the complementary expertise and experience of Pherecydes Pharma and the CEA. The aim of this three-year project is to offer new treatments for hard-to-treat or resistant E. Coli infections thanks to precision phagotherapy. To provide the best approach to the therapeutic need, Pherecydes Pharma and the CEA will develop a new generation of tools to measure the efficacy of anti-E. Coli phages on the patient’s bacterial strain. These new tools could then become the basis for a brand-new generation of phagograms1 for the various target bacteria. This financial support will also allow Pherecydes Pharma to accelerate the development of its anti-E. coli phages.
6
Favorable opinion from the institutional review board (CPP) to initiate the PhagoDAIR study
On February 7, 2022, the Company announced that it had received a favorable opinion from the Ile de France III CPP for the PhagoDAIR study, a phase II pilot clinical study in the treatment of osteoarticular infections of prostheses caused by Staphylococcus aureus.
PhagoDAIR is the world’s first phagotherapy study conducted in this indication. Its protocol was already approved by the ANSM in December 2021.
It will be conducted in France, Spain and other European countries (Germany and the Netherlands) on 64 patients with a knee or hip joint infection due to S.-aureus, divided into one group who will be treated with phagotherapy and a control group who will receive a placebo, in addition to the standard of care. The patients treated with phagotherapy will receive anti-Staphylococcus aureus phages that are active on their strain, selected thanks to Pherecydes Pharma’s phagogram. The standard of care will comprise the surgical procedure called DAIR (Debridement, Antibiotics, Implant Retention) combined with a suppressive antibiotic therapy. An assessment will be carried out 12 weeks after the phagotherapy, and patients will continue to be monitored for two years.
The favorable opinion of the CPP paves the way for the start of enrollment in the study at French centers in spring 2022. Results are expected in the summer of 2023 and follow-up will continue until the first half of 2025. Depending on the preliminary results of phase I/II, Pherecydes Pharma will conduct a phase III study on the same indication, which could start in late 2024.
Pherecydes Pharma announces a change in its governance structure to become a company with a Board of Directors
On February 24, 2022, the Company announced it was changing its governance structure by adopting a board of directors and general management instead of the existing Supervisory Board and Management Board.
The setting up of a board of directors and general management corresponds to Pherecydes Pharma’s wish to simplify its operating decision procedures and more readily respond to the rapidity, efficacy and reactivity requirements specific to the biotechnology sector while ensuring the continuity of the Company’s leadership and administration, with the mandates of the current representatives having been renewed. The Company will thus be able to confidently address the next decisive stages of its activity, in particular the implementation of the PhagoDAIR clinical study.
As a result, the ordinary and extraordinary general meeting of the shareholders, which was held on May 19, 2022, was asked to:
| • | modify the Company’s method of management and administration by adopting a board of directors and general management; |
| • | correspondingly amend the Company’s articles of association; |
7
| • | appoint the current members of the supervisory board as the first members of the board of directors. |
Following this General Meeting of Shareholders, the Board of Directors decided on general management’s method of exercise. The newly formed Board of Directors was thus asked to:
| • | approve the separation of the functions of the President of the board of directors and the chief executive officer; |
| • | appoint Mr. Guy-Charles Fanneau de La Horie, the current President of the management board, as chief executive officer; and |
| • | appoint Didier Hoch, the current President of the Supervisory Board, as President of the board of directors. |
The separation of the functions of President of the board of directors and chief executive officer is in line with the Autorité des marchés financiers‘s (AMF) 2021 report on “corporate governance and the executive compensation of listed companies” prepared pursuant to Article L.621-18-3 of the French Monetary and Financial Code.
The Company also announced that Philippe Rousseau, Chief Operating Officer, and a member of the management board, left the Company to pursue other ventures.
Pherecydes Pharma extends its intellectual property in China with a first patent granted for its anti-Pseudomonas aeruginosa phages in this territory
On March 8, 2022, the Company announced the granting of a patent for its anti-Pseudomonas aeruginosa phages by the Chinese patent office.
This second patent granted in China, following the one granted the preceding December for its anti-Staphylococcus aureus phages, gives the Company protection until the end of 2034 on this vast market.
Pherecydes Pharma and BIOASTER join forces in the treatment of bacteremia
On March 11, 2022, the Company and BIOASTER, the first Institute for Technological Innovation in Microbiology, announced the implementation of research collaboration to use phages to treat bacteremia (blood infections) caused by the S. aureus, E. Coli and Pseudomonas aeruginosa bacteria.
Bacteremia, defined as the presence of viable bacteria in the bloodstream, represents the world’s second greatest cause of bacterial infection, with around 1.5 million deaths associated with this pathology per year In the United States alone, the Centers for Disease Control and Prevention (CDC) estimates that up to 1.7 million people develop bacteremia every year. S. aureus is the most commonly identified pathogenic agent, both within and outside a hospital setting. Age-adjusted mortality assessments show that mortality due to S. aureus bacteremia is higher than that of AIDS, tuberculosis, or viral hepatitis and similar to the mortality rate of breast or prostate cancer. E. Coli and Pseudomonas aeruginosa also represent major causes of antibiotic-resistant bacteremia.
8
Within the framework of this collaboration, Pherecydes Pharma will draw on BIOASTER’s excellence in developing preclinical models that are relevant to these infections to test the efficacy of the proprietary phages selected by Pherecydes Pharma in these pathologies.
Pherecydes Pharma obtains a new patent for its anti-Pseudomonas aeruginosa phages in Europe
On March 22, 2022, the Company announced the granting of a new patent for its anti-Pseudomonas aeruginosa phages by the European Patent Office (EPO).
This new patent granted in Europe gives the Company protection until 2034 on this vast market.
Pherecydes Pharma announces a partnership with Navarrabiomed to evaluate its anti-S. aureus phages in a new indication
On March 31, 2022 Pherecydes Pharma announced the signing of a research agreement with Navarrabiomed to evaluate its anti-S. aureus phages in the treatment of endocarditis.
Endocarditis is an infection of the endocardium (inner layer of the heart), the heart valves or the aorta, which inevitably leads to hospitalization. This infection is most often caused by bacteria, mainly Staphylococcus aureus. This bacterium is among the six most deadly germs1 in antibiotic resistance and is on the WHO priority pathogens list2 for R&D of new antibiotics.
The objective of the Navarrabiomed research team is to test Pherecydes Pharma’s phages on a porcine model of S. aureus endocarditis.
This is the third indication targeted by the company’s anti-Staphylococcus aureus phages, after bone and joint infections of prostheses and diabetic foot ulcers.
Pherecydes Pharma announces the appointment of Thibaut du Fayet as Chief Operating Officer and member of the Management Board
On April 7, 2022 Pherecydes Pharma announced that Thibaut du Fayet, an expert in the biotech sector, joined the Management Board of Pherecydes Pharma as Chief Operating Officer.
Thibaut du Fayet brings to the company over 20 years of experience in the pharmaceutical and life sciences industry. After obtaining an MBA from ESSEC Business School in 1992, he was Senior Managing Director at Bossard-Gemini for six years. From 2000, he held management positions first at Rhône Poulenc as Strategy Director for four years, and then at BioMerieux where he was also Strategic Marketing Director for four years. In 2008, he joined Transgene where he held the position of VP Corporate Development, Alliances & Programs Management for 14 years.
Through his various leadership roles in development, strategy, business development, and program management, he has acquired important and diverse skills in the pharmaceutical industry.
| 1 | Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis; The Lancet, January 19, 2022 |
| 2 |
https://www.who.int/fr/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed |
9
Pherecydes Pharma announces the first international approval of a compassionate treatment with its phages – The Swedish Medical Products Agency (SMPA) has given its approval to treat a case of osteoarticular infection of a prosthesis with the Company’s anti-S. aureus phages
On April 28, 2022, Pherecydes Pharma announced the first international approval of a compassionate treatment with its phages, in Sweden.
The Swedish Medical Products Agency (SMPA) has given its approval to treat a case of osteoarticular infection of a prosthesis with Pherecydes Pharma’s anti-S. aureus phages.
With the compassionate treatments that continue to be provided in France, Pherecydes Pharma has now treated more than 75 patients in this context.
The general meeting of the shareholders of Pherecydes Pharma approves the change in its governance to a company with a Board of Directors – The new Board of Directors has appointed Didier Hoch as Chief Executive Officer and Thibaut du Fayet as Chief Operating Officer
On May 23, 2022, Pherecydes Pharma announced that the Combined General Meeting of Shareholders (CGM) held on May 19, 2022 was able to deliberate, with the quorum having been reached.
The CGM adopted all the proposed resolutions, except for the twenty-third resolution which was rejected, in accordance with the Board’s recommendations.
In particular, the company’s shareholders approved the change in the company’s method of management and administration by adopting a Board of Directors and general Management.
The current members of the Supervisory Board were appointed as the first directors of Pherecydes Pharma.
The Board of Directors is thus composed of seven members, two of whom are independent.
At the end of the general meeting of shareholders, the Board of Directors adopted the following decisions:
| • | the Board of Directors has taken note of the termination of the functions of Mr. Guy-Charles Fanneau de La Horie, previously President of the Management Board, who did not wish, for personal reasons, to assume the mandate of Chief Executive Officer in the new governance; |
| • | consequently, and contrary to what had been announced, the Board has not decided to adopt, at this stage, a separation of the functions of Chief Executive Officer and President of the Board of Directors; |
| • | Mr. Didier Hoch, previously President of the Supervisory Board, was appointed Chief Executive Officer; and |
| • | Mr. Thibaut du Fayet, previously a member of the Management Board, was appointed Chief Operating Officer. |
10
The Board of Directors thanked Mr. Guy-Charles Fanneau de La Horie who has been one of the driving forces in the development of the company, both operationally and financially, notably with the company’s successful IPO in 2021. Mr. Guy-Charles Fanneau de La Horie will continue to contribute his expertise, on a transitional basis, within the framework of a service contract.
There are also plans to adopt, at a later stage, a separation of the functions of Presidentof the Board of Directors and Chief Executive Officer, and thus to appoint a new Chief Executive Officer.
Pherecydes Pharma obtains Early Access Approval (Autorisation d’Accès Compassionnel—AAC) from the ANSM for its anti-Staphylococcus aureus phages – This is the first AAC for a treatment in patients who have failed antibiotic therapy, particularly expected in the context of the fight against antibiotic resistance.
On May 30, 2022, Pherecydes Pharma announced that it had been granted Early Access Approval (AAC) from the ANSM for its anti-Staphylococcus aureus (S. aureus) phages.
The new AAC system (formerly nominative authorization for early access, or ATU), introduced in July 2021, allows certain categories of sick patients in France with no therapeutic solutions to benefit from drugs yet to be granted marketing approval (Autorisation de mise sur le marché – AMM).
As of May 30, 2022, Pherecydes Pharma had treated more than 60 patients as part of compassionate treatment under the supervision of the ANSM, excluding AAC status. These phages, administered by various routes (intra-articular, intravenous, bronchoalveolar nebulization, etc.), have demonstrated excellent tolerance with no reported side effects.
The AAC scheme allows Pherecydes Pharma to make its anti-S. aureus phages available to larger populations and thus generate the first revenue in the Company’s history.
Pherecydes Pharma announces the enrollment of the first patient in the PhagoDAIR phase II study in the treatment of osteoarticular infections of prostheses caused by Staphylococcus aureus.
On June 15, 2022, Pherecydes Pharma announced the enrollment of the first patient in the PhagoDAIR phase II clinical trial.
PhagoDAIR is the world’s first study of phagotherapy conducted in osteoarticular infections of prostheses caused by Staphylococcus aureus (S. aureus). Its protocol was approved by the ANSM in December 2021 and by the CPP in February 2022.
Since then, Pherecydes Pharma has been actively recruiting patients for this clinical trial, the first of which has just been enrolled in France at the Hospices Civils de Lyon (Hospices Civils de Lyon—HCL).
The study, also conducted in other European countries, includes 64 patients with a knee or hip joint infection due to S. aureus, divided into one group treated with phagotherapy and a control group receiving a placebo, in addition to the standard of care. The patients treated with phagotherapy receive anti-S. aureus phages that are active on their strain, selected thanks to Pherecydes Pharma’s phagogram.
11
The standard of care comprises the surgical procedure called DAIR (Debridement, Antibiotics, Implant Retention) combined with a suppressive antibiotic therapy. An assessment is carried out 12 weeks after the phagotherapy, and patients continue to be monitored for two years. Follow-up of patients continues until the first half of 2025.
Depending on the preliminary results of phase II, Pherecydes Pharma will conduct a phase III study on the same indication, which could start in 2024.
Positive pre-clinical results for inhaled phagotherapy presented to the Reanimation 2022 conference – A pre-clinical study demonstrates the benefits of inhaled Pherecydes Pharma phages in treating mechanical ventilation-associated pneumonia
On June 27, 2022, Pherecydes Pharma announced that the results of a pre-clinical study conducted with its phages were presented at the Reanimation 2022 conference organized by the French Intensive Care Society (Société de Réanimation de Langue Française – SRLF) and held in Paris from June 22 to 24, 2022.
Antoine Guillon, an intensive care doctor at the University Hospital of Tours and researcher at the Research Center for Respiratory diseases at the University of Tours (Étude des Pathologies Respiratoires Inserm-Université de Tours – UMR1100), presented the results of the study during a Flash Com session entitled “Inhaled bacteriophage therapy in a porcine model of pneumonia caused by Pseudomonas aeruginosa during mechanical ventilation.”
The study was carried out within the framework of the Pneumophage project, associating the UMR1100 and the company Diffusion Technique Française, aimed at demonstrating the effectiveness of inhaled phagotherapy in treating mechanical ventilation-associated infections. To this end, the UMR1100 researchers in Tours developed a porcine model of pneumonia caused by P. aeruginosa incorporating the essential characteristics of the human disease. The work undertaken showed that the use of Pherecydes Pharma’s anti-P. aeruginosa phages significantly reduced the lung bacterial load (1.5-log reduction, p < 0.001).
The results obtained also demonstrate:
| • | the feasibility of delivering large quantities of active phages by nebulization during mechanical ventilation; |
| • | the rapid control of the infection in situ in a respiratory model close to humans. |
This demonstration in a highly translational model paves the way for pulmonary phagotherapy, notably in intensive care units where the occurrence of mechanical ventilation-associated P. aeruginosa infections is higher than in other units. The P. aeruginosa bacterium is a major cause of mechanical ventilation-associated pneumonia, a pathology with a relapse rate of 40% and a high mortality rate of around 20%.
12
Pherecydes Pharma announces an initial registration of its phagogram as an in vitro diagnostic test in accordance with EC directives – This vital tool for precision medicine thus meets regulatory requirements and clinicians’ expectations
On September 12, 2022, Pherecydes Pharma announced the registration of its phagogram as an in vitro diagnostic test (“Phagogram 1.5”) in accordance with Directive 98/79/EC.
The phagogram is an in vitro diagnostic test to verify the sensitivity of patients’ bacterial strains to Pherecydes Pharma’s phages.
Pherecydes Pharma is developing its phagogram for three target bacteria: Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. These bacteria, which are the most resistant and dangerous according to the WHO, alone account for more than two thirds of hospital-acquired resistant infections in industrialized countries3.
The EC registration of the Phagogram 1.5 was made possible by the setting up of a new specialized laboratory in Nantes; this new laboratory initially ensured the development and validation of the in vitro diagnostic test following a very stringent process from a technical, quality, and regulatory perspective. It is now responsible for carrying out phagograms for patients enrolled in our clinical studies and patients undergoing compassionate treatment.
The Phagogram 1.5 program is the first stage in a broader strategic program whose goal is to develop a new generation phagogram, “Phagogram 2.0,” in collaboration with the CEA (see Pherecydes Pharma press release from January 3, 2022). This program aims to significantly shorten the lead time required for a diagnosis and thus offer phagotherapy in all types of indications, whether acute or chronic.
Pherecydes Pharma announces the creation of an International Medical Advisory Board – This group of infectious disease experts will contribute to the design and implementation of the Company’s clinical development strategy in phagotherapy
On September 15, 2022, Pherecydes Pharma announced the establishment of a Medical Advisory Board comprising prominent international scientific and clinical experts in infectious diseases.
This Board will support Pherecydes Pharma in consolidating its clinical development strategy in phagotherapy. It will meet twice a year, with its first meeting on September 28, 2022.
The members of the Medical Advisory Board are:
| • | Dr. Saima Aslam, Associate Professor of Medicine, Infectious Disease Specialist at UC San Diego, California, USA. |
| • | Prof. Marc Bonten, President of the Infection & Immunity strategic research program at UMC Utrecht, the Netherlands. |
| • | Prof. Tristan Ferry, Deputy Head of the Infectious and Tropical Diseases department, Hospices Civils de Lyon, and President of the national CRIOAc scientific committee, France. |
| • | Prof. Yok-Ai Que, Associate Professor in Intensive Care Medicine at Bern University Hospital, Switzerland. |
| • | Prof. Martin Witzenrath, Head of Department of Respiratory Diseases and Intensive Care at the Charité Universitätsmedizin Berlin, Germany. |
| 3 | Opatowski M—Hospitalisations with infections related to antimicrobial-resistant bacteria from the French nationwide hospital discharge database, 2016 |
13
Pherecydes Pharma successfully completes €3.1 million fundraising
On September 22, 2022, Pherecydes Pharma announced the success of its capital increase for a total amount of €3.1 million, comprising €2.6 million from institutional investors and €0.5 million from individuals.
The Company plans to use the net proceeds of this fundraising to partially finance:
| 1) | for around 50%, the clinical development of anti-Staphylococcus aureus (S. aureus) phages notably including: |
| • | the ramping up of the PhagoDAIR phase II study in France and Europe with the opening of new clinical centers, mainly in Spain and the Netherlands, through to the key criterion assessment stage; |
| • | the preparation and initiation of a Phase II study in a clinical indication with substantial medical needs; |
| • | the launch and management of studies (PHRC) for which the Company is not the sponsor (PhagoPied, Phagos) via the conducting of Phagograms and various associated costs; |
| 2) | for around 30%, the development of anti-Pseudomonas aeruginosa phages notably including: |
| • | the initiation of a regulatory toxicology study; |
| • | the preparation and initiation of a (phase II) clinical study in an indication associated with respiratory infections with substantial medical needs; |
| 3) | for around 10%, perfecting manufacturing and quality control processes, producing GMP batches of E. Coli phages and completing preclinical development; |
| 4) | for around 10%, research work associated with the identification of a new bacterial target, as well as the Company’s other running costs. |
Pherecydes Pharma announces the positive recommendation of the DSMB for the continuation of its phase II clinical study PhagoDAIR in osteoarticular infections of prostheses caused by Staphylococcus aureus
On November 29, 2022, Pherecydes Pharma announced that it had received a unanimous recommendation from the Data Safety Monitoring Board (DSMB) to continue without modification the phase II clinical study PhagoDAIR in osteoarticular infections of prostheses due to Staphylococcus aureus (S. aureus).
The DSMB is an independent expert committee responsible for the open-label review of the PhagoDAIR study safety data and will meet twice a year during the study. Following its first meeting, the committee recommended the continuation of the study without modification.
Pherecydes Pharma: Minutes of the Combined General Meeting of Shareholders
On December 15, 2022, Pherecydes Pharma announced that the Combined General Meeting of the shareholders (CGM) was able to deliberate, with the quorum having been reached. The shareholders followed all the voting recommendations of the Board of Directors.
14
Following the Meeting, the Board of Directors adopted the following decisions:
| • | The functions of Chief Executive Officer and President of the Board of Directors were separated as planned following the CGM on May 19, 2022; |
| • | Mr. Thibaut du Fayet, previously Chief Operating Officer, was appointed Chief Executive Officer; |
| • | Mr. Didier Hoch, previously President and Chief Executive Officer, was appointed President of the Board of Directors. |
| 1.3 | RESULTS OF THE ACTIVITY, PROGRESS MADE AND DIFFICULTIES ENCOUNTERED |
| 1.3.1 | Economic and financial results |
| Simplified income statement (in euros) |
2022 | 2021 | ||||||
| Operating income |
3,167,235 | 2,200,380 | ||||||
| Operating expenses |
9,857,278 | 6,521,075 | ||||||
| Operating results |
(6,690,043 | ) | (4,320,694 | ) | ||||
| Financial results |
(63,504 | ) | (46,346 | ) | ||||
| Extraordinary results |
511,125 | 189,470 | ||||||
| Net results (loss) |
(4,854,342 | ) | (3,189,057 | ) | ||||
On December 31, 2022, total operating income, essentially consisting of capitalized production associated with the Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) programs, was €3,167 thousand compared with €2,200 thousand at December 31, 2021, an increase of 44%. This growth was notably a result of the increase in R&D activity associated with these programs between the two years in question.
On December 31, 2022, operating expenses totaled €9,857 thousand over the year, up 50% from the fiscal year 2021 figure of €6,521 thousand. This growth was essentially due to the 125% increase in subcontracting charges to €2,357 thousand in 2022, and reflects the expenses related to the subcontracting of the PhagoDAIR phase II clinical trial.
The annual operating loss was €6,690 thousand as of December 31, 2022 versus a loss of €4,321 thousand at December 31, 2021, an increase of 55% between the two years, primarily as a result of the increase in operating expenses from one year to the next.
Exceptional results totaled €551 thousand at December 31, 2022 compared with €189 thousand the previous year, reflecting income from management operations related to debt write-off on the PHAGOSCLIN project.
The Research Tax Credit amounted to €1,384 thousand at December 31, 2022 versus €989 thousand the previous year.
Net loss stood at €4,854 thousand in 2022 compared with a loss of €3,189 thousand the previous year.
15
Financial structure and cash position
On December 31, 2022, the Company had a cash position of €1,035 thousand, versus €5,357 thousand at December 31, 2021. Shareholders’ equity stood at €7,522 thousand on December 31, 2022 compared with €9,228 thousand at December 31, 2021.
On September 22, 2022, Pherecydes Pharma successfully carried out a capital increase of €3.1 million, comprising €2.6 million from institutional and historical investors and €0.5 million from individuals, via the PrimaryBid platform.
On February 17, 2023, Pherecydes Pharma announced the completion of the capital increase announced on February 15, 2023 in connection with the publication of its proposed merger with the company ERYTECH (see press release from February 15, 2023 and section 1.7). This capital increase for €1.5 million was fully subscribed by Auriga IV Bioseeds, Ouest Ventures III and the pool of shareholders represented by Guy Rigaud, historical shareholders of Pherecydes Pharma.
The funds raised through this capital increase will provide Pherecydes Pharma with sufficient resources to finance its cash requirements until the completion of the merger expected to take place no later than June 30, 2023.
| 1.3.2 | Results for the fiscal year ended December 31, 2022 |
The Company’s result for the year was a loss of €4,854,342.477.
| 1.4 | RESEARCH AND DEVELOPMENT ACTIVITIES |
The Company has developed research, pre-clinical and clinical testing programs to develop optimized bacteriophages to combat three prevalent antibiotic-resistant diseases.
The importance of its research and development activity is reflected in the predominance in the Company’s workforce of employees with high-level scientific training and dedicated to laboratory work.
As of December 31, 2022, 28 of the company’s employees are involved in research and development, representing 90% of the total workforce at that date.
| 1.5 | POLLUTING OR RISKY ACTIVITIES |
Pursuant to the provisions of Article L.225-102-2 of the French Commercial Code, below you will find a description of polluting or risky activities.
The Company does not operate a classified facility and is therefore not subject to regulations on classified facilities and technological risks, but it does use hazardous chemical and biological products. Regular inspections are carried out on the Company’s laboratories. Although the Company believes that it meets its current legal obligations relating to the environment, non-compliance would expose it to criminal and administrative penalties, including the suspension or revocation of authorizations and approvals required for its activities.
16
Since the creation of the Company, no incidents have occurred in the context of polluting or risky activities. The Company therefore believes that it continues to meet the legal obligations relating to these activities in force as of the date of this report.
Compliance with applicable environmental, health and safety regulations imposes costs on the Company and may require significant investment, particularly in the future if regulatory changes require the use of new equipment or procedures.
In addition, although the Company believes that the safety procedures it implements for the storage, use, transportation and disposal of hazardous, chemical, and biological products and industrial and hospital waste are in compliance with applicable regulations, the risk of accidents or accidental contamination cannot be eliminated. In the event of an accident or contamination, the Company could be held liable, which could result in it incurring potentially significant costs to compensate the victims and remedy the damage.
| 1.6 | MAIN RISKS AND UNCERTAINTIES FACING THE COMPANY AND FINANCIAL RISK MANAGEMENT |
| 1.6.1 | Liquidity risks |
Since its founding, the Company has financed its growth by strengthening its equity through successive capital increases, loans, and obtaining public funding for innovation (grants, research tax credit, etc.).
Significant expenses have been incurred in relation to the research and development of the Company’s products and to obtaining the various regulatory approvals for their marketing. To date, this has resulted in negative cash flows from operating activities.
As of December 31, 2022, loans and debt amounted to €2.6 million, comprising:
| • | a €2.2 million government-backed loan, the first repayment of which will be made in July 2023 followed by quarterly repayments for four years; and |
| • | an innovation (R&D) loan of €0.4 million granted by BPI France in October 2020 and repaid quarterly over five years since June 2022. |
| 1.6.2 | Risks related to historical and future losses |
Since its founding at the end of 2006, the Company has recorded operating losses every year. These losses are mainly due to research and development costs, in particular the costs of strengthening the research and development teams, incurred by the Company to develop its technologies.
If the Company fails to achieve sufficient revenue growth over the next few years, it could experience further operating losses, including due to:
| • | Research and development activities, mainly; |
| • | Marketing and development costs for its products; |
| • | Its international development. |
A significant increase in these expenses could have a material adverse effect on the Company, its business, financial position, results, development, or outlook.
17
If the Company fails to generate taxable income, it will not be able to use the tax loss carryforwards generated by the Company since its founding, totaling €24,087,822 at the closing date of fiscal year 2022.
| 1.6.3 | Risks of impairment of intangible assets |
As of December 31, 2022, intangible assets represented a net amount of €9,072 thousand, and the net balance sheet total at that date stood at €13,216 thousand.
As of December 31, 2022, the Company has capitalized research and development expenses for a gross value of €5,555 thousand. The capitalized development costs relate to clearly individualized subjects with a high probability of technical success and commercial profitability or economic viability. Pherecydes Pharma has considered as eligible for capitalization the development in-progress costs of products for which the regulatory authorities have validated the Company’s good manufacturing practices or have given the Company authorization to proceed with human clinical trials and/or Early Access Approval (AAC). The net costs capitalized as assets under construction amount to €5,074 thousand. They mainly correspond to development costs for various assets:
| • | Pseudomonas aeruginosa for €547 thousand; |
| • | Staphylococcus aureus for (€5,602) thousand. |
Other intangible assets correspond to patents for an amount of €123 thousand on December 31, 2022. The Company has four patent families (two for anti-Pseudomonas aeruginosa phages, one for anti-Staphylococcus aureus phages and one for anti-E. coli phages). These patents have been granted in some jurisdictions and are pending in others.
In accordance with accounting methods, these intangible assets are recorded at their initial acquisition cost minus accumulated amortization (straight-line amortization over 20 years) and, where applicable, impairment losses.
The change in value of the Company’s intangible assets depends on its development plan and the success or failure of its programs. Should the ANSM decide to not grant AACs for the anti-Pseudomonas aeruginosa phages, or if it becomes impossible for the Company to make progress on these phages during the clinical development process, it would result in an impairment of these intangible assets. The Company would then have to review the value of these assets and record an impairment corresponding to the total value or a partial amount of these assets. Each program will need to be reviewed independently as part of this evaluation: the failure of one program does not automatically mean the failure of the other. An impairment of these assets could have a material financial impact, resulting in a decrease in the size of the balance sheet and a significant loss on the income statement.
| 1.6.4 | Risks relating to shares and other financial instruments |
The Company does not hold any equity investments or marketable securities.
18
| 1.6.5 | Dilution risks |
As part of its employee profit-sharing policy, the Company has regularly issued financial instruments giving access to its capital (start-up stock options, free shares, etc.).
At December 31, 2022, the following dilution would result if holders were to exercise these securities giving access to capital:
| Number of shares comprising the share capital |
In the event of all start-up stock options being exercised |
In the event of the definitive acquisition of all the free shares allocated |
TOTAL | |||||||||||||
| Number ofshares created |
7,221,477 | 615,450 | 58,523 | 673,973 | ||||||||||||
| Share capital after issue of new shares |
N/A | 7,836,927 | 7,280,000 | 7,895,450 | ||||||||||||
| Dilution |
N/A | 7.85 | % | 0.80 | % | 8.54 | % | |||||||||
As part of its policy of motivating its employees and corporate officers, the Company may issue new financial instruments giving access to its capital in the future.
In addition, in the context of financing its business development, the Company may issue or be required to issue new securities on the market and thus increase its share capital to finance all or part of its operating needs.
The issue of these financial instruments or new shares would result in additional, potentially significant dilution for the Company’s shareholders.
| 1.7 | SIGNIFICANT EVENTS BETWEEN THE BALANCE SHEET DATE AND THE REPORTING DATE |
ERYTECH and PHERECYDES announce a strategic combination to create a global leader in phagotherapy
On February 15, 2023, Pherecydes Pharma and ERYTECH Pharma (“ERYTECH”; Nasdaq & Euronext: ERYP), a clinical-stage biopharmaceutical company developing innovative therapies by encapsulating therapeutic drug substances inside red blood cells, announced their proposed strategic combination intended to create a global player in extended phagotherapy and accelerate the development of a portfolio of drug candidates targeting pathogenic bacteria and potential other indications of high unmet medical needs.
The strategic combination would build on the complementary expertise and capabilities of both companies to accelerate the development of extended phagotherapies for antimicrobial resistance, in particular via the PhagoDAIR phase II study conducted by PHERECYDES, as well as other anti-infective fields and therapeutic areas with high unmet medical needs.
19
The cash runway of the new entity resulting from the merger would extend into the third quarter of 2024, with a consolidated cash position of approximately €41 million as of December 31, 2022, and would enable funding of existing and novel programs through multiple clinical milestones.
This transaction would pave the way for the expansion of PHERECYDES development programs in antimicrobial resistance and complete the strategic review process announced by ERYTECH on several occasions since November 2021.
The transaction expected to close at the end of the second quarter of 2023.
The transaction, supported by the key shareholders of PHERECYDES and ERYTECH, will result in former PHERECYDES shareholders holding approximately 49% of the combined entity.
ERYTECH and PHERECYDES intend to merge their operations and relocate all teams to ERYTECH’s premises in Lyon, France, where they will benefit from being located within a major European center for infectious diseases.
Following the announcement of its proposed merger with Erytech, Pherecydes Pharma completes a €1.5 million capital increase reserved to its historical shareholders
On February 17, 2023, Pherecydes Pharma announced the completion of the capital increase announced on February 15, 2023 in connection with the publication of its proposed merger with the Company Erytech.
This capital increase for €1.5 million was fully subscribed by Auriga IV Bioseeds, Ouest Ventures III and the pool of shareholders represented by Guy Rigaud, historical shareholders of Pherecydes Pharma.
The funds raised through this capital increase will provide Pherecydes Pharma with sufficient resources to finance its cash requirements until the completion of the merger expected to take place no later than June 30, 2023.
| 1.8 | FORESEEABLE DEVELOPMENTS AND OUTLOOK |
During fiscal years 2023–2024, the Company intends to complete its merger with ERYTECH, pursue the development of its various assets and, accelerate the commercial ramp-up of precision phagotherapy.
Completion of the merger with ERYTECH Pharma to become a global leader in phagotherapy
As of today’s date, the memorandum of understanding has been unanimously approved by the Boards of Directors of ERYTECH and PHERECYDES.
The Extraordinary General Meetings of Shareholders (“EGM”) of ERYTECH and PHERECYDES will be called to vote on the Proposed Merger. Said meeting is currently expected to be held at the end of the first half of 2023 (or at the beginning of the second half of 2023).
20
The resolutions to approve the Proposed Merger will require, within each company, the positive vote of two thirds of the shareholders present or represented. The ERYTECH EGM will also be called to approve the name change of the new entity, to mark the beginning of a new development stage for the combined activities.
Ambitious clinical and pre-clinical development strategy
| • | Expansion of the PhagoDAIR phase II study and preparation for phase III |
The Company plans to expand its ongoing PhagoDAIR phase II study in patients with knee and hip joint infections due to Staphylococcus aureus (S. aureus) by opening new clinical centers in Europe, with results expected in the first quarter of 2024.
The Company will also prepare the protocol for the phase III study that will follow the completion of the PhagoDAIR phase II study.
| • | Expansion of Pherecydes’ clinical phagotherapy portfolio |
The Company plans to expand its clinical portfolio with two additional phase II studies funded by the Company, one in patients with S. aureus endocarditis, which is expected to begin in mid-2023, and the second in patients with complex urinary tract infections due to Escherichia coli (E. coli), which is expected to begin in the first quarter of 2024. The Company plans to potentially open clinical centers in the United States for these two studies.
| • | Creation of a research and development strategy based on ERYTECH Pharma platforms and expertise |
In particular, the Company plans to develop drug-delivery solutions using red blood cells or red blood cell-derived vesicles from ERYTECH Pharma’s platforms (the ERYCAPS and ERYCEV platforms, respectively).
The Company also intends to leverage ERYTECH Pharma’s formulation and oncology expertise to support phage- and endolysin-based therapeutic approaches, drawing on Erytech’s know-how in protein engineering and in anti-infective fields such as antimicrobial resistance and beyond (such as food, cosmetics, and animal health), or the development of novel carriers.
| • | Expansion of the PHERECYDES portfolio |
The Company plans to expand its portfolio with two new phages, complementary to the three already existing (S. aureus, P. aeruginosa, E. Coli). These phages are critical in developing a comprehensive target portfolio in the fight against resistant bacterial infections.
International development and strong financial visibility
The Company plans to open new clinical study centers in Europe for the PhagoDAIR phase II clinical study, including Spain, the Netherlands, Germany, and the United Kingdom.
21
The Company intends to strengthen its Scientific and Clinical Committee with new members of an international caliber.
The Company will also continue its efforts with the European Medicine Agency (EMA) and the US Food and Drug Administration (FDA).
The Company will capitalize on ERYTECH’s presence in the United States to facilitate access to North American clinical and regulatory players with a view to its future phase II and III clinical developments. This presence in the United States will benefit the Company’s financial visibility with US investors.
International industrial strategy
In the medium term, the Company will seek to consolidate its strategic industrial partnerships and establish complementary industrial solutions to protect itself against the risk of clinical batch supply shortages.
At the same time, the Company will pursue an international industrial roll-out strategy to support its global development, in line with its clinical development.
22
CHAPTER 2— SUBSIDIARIES AND EQUITY INVESTMENTS
| 2.1 | SUBSIDIARIES OF THE COMPANY |
As of December 31, 2022, the Company has no subsidiaries.
| 2.2 | ACQUISITION OF SIGNIFICANT SHAREHOLDINGS IN COMPANIES HEADQUARTERED IN FRANCE OR ACQUISITION OF CONTROL OF SUCH COMPANIES |
In accordance with Article L.233-6 of the French Commercial Code, we hereby inform you that during the fiscal year ended December 31, 2022, the Company did not make any equity investments in any company with its registered office in France.
23
CHAPTER 3— INFORMATION ON PAYMENT TERMS TO SUPPLIERS AND CUSTOMERS
Below is a table showing the balance of the Company’s trade account payables and receivables by due date as of December 31, 2022, in accordance with Articles L.441-14 and D.441-6 of the French Commercial Code:

24
CHAPTER 4— TOTAL DIVIDENDS DISTRIBUTED OVER THE LAST THREE YEARS
The Company has not distributed any dividends in the last three fiscal years.
The Company plans to use all available funds to finance its activities and growth, and therefore does not intend to distribute dividends in the near future.
25
CHAPTER 5— FIVE YEAR SUMMARY OF RESULTS
Below you will find the five year summary of results required by Article R.225-102 para. 2 of the French Commercial Code:
| Breakdown of Indications/Periods |
12/31/2022 | 12/31/2021 | 12/31/2020 | 12/31/2019 | 12/31/2018 | |||||||||||||||
| Duration of fiscal year |
12 months | 12 months | 12 months | 12 months | 12 months | |||||||||||||||
| I—Financial situation at the end of the fiscal year |
| |||||||||||||||||||
| a) Share capital |
7,211,477 | 5,852,308 | 4,230,687 | 3,293,694 | 2,784,042 | |||||||||||||||
| b) Number of shares issued |
7,221,477 | 1,621,621 | ||||||||||||||||||
| c) Number of bonds convertible into shares |
||||||||||||||||||||
| II—Overall results of actual operations |
||||||||||||||||||||
| a) Revenue excluding taxes |
||||||||||||||||||||
| b) Income before taxes, depreciation, amortization and provisions |
5,797,999 | -3,949,499 | -1,996,472 | -1,974,371 | -1,447,205 | |||||||||||||||
| c) Income tax |
-1,388,080 | -988,514 | -413,732 | -1,028,138 | -367,344 | |||||||||||||||
| d) Income after taxes but before depreciation, amortization and provisions |
-4,409,919 | -2,960,985 | -1,582,740 | -946,233 | -1,079,861 | |||||||||||||||
| e) Income after taxes, depreciation, amortization and provisions |
-4,854,342 | -3,189,057 | -1,395,340 | -1,221,868 | -2,419,726 | |||||||||||||||
| f) Amount of distributed income |
||||||||||||||||||||
| g) Employee shareholding |
||||||||||||||||||||
| III—Results of operations per share |
||||||||||||||||||||
| a) Income after taxes but before depreciation or amortization |
||||||||||||||||||||
| b) Income after taxes, depreciation, amortization and provisions |
||||||||||||||||||||
| c) Dividend paid to each share |
||||||||||||||||||||
| IV—Personnel: |
| |||||||||||||||||||
| a) Number of employees |
||||||||||||||||||||
| b) Payroll amount |
2,330,672 | 2,015,555 | 1,341,644 | 1,600,316 | 1,151,093 | |||||||||||||||
| c) Amounts paid for employee benefits |
990,671 | 893,130 | 578,951 | 693,375 | 514,182 | |||||||||||||||
26
CHAPTER 6—BREAKDOWN OF SHARE CAPITAL AND TREASURY SHARES
| 6.1 | BREAKDOWN OF SHARE CAPITAL DURING THE LAST FISCAL YEAR |
The table below shows the breakdown of the Company’s capital and voting rights as of December 31, 2022:
| Number of shares and voting rights at December 31, 2022 | ||||||||||||||||
| Shareholders |
Shares held | Percentage of capital |
Number of voting rights |
Percentage of voting rights |
||||||||||||
| Members of the Board of Directors and general management |
4,483 | 0.06 | % | 4,483 | 0.06 | % | ||||||||||
| Elaia Capital |
1,491,037 | 20.65 | % | 1,491,037 | 20.65 | % | ||||||||||
| Go Capital |
1,023,103 | 14.17 | % | 1,023,103 | 14.17 | % | ||||||||||
| Tikehau ACE Capital |
1,384,564 | 19.17 | % | 1,384,564 | 19.17 | % | ||||||||||
| Omnes Capital |
301,494 | 4.17 | % | 301 ,494 | 4.17 | % | ||||||||||
| Participations Besancon |
243,819 | 3.38 | % | 243,819 | 3.38 | % | ||||||||||
| GR Pool |
484,645 | 6.71 | % | 484,645 | 6.71 | % | ||||||||||
| Treasury shares |
22,057 | 0.31 | % | 22,057 | 0.31 | % | ||||||||||
| Public |
2,266,275 | 31.38 | % | 2,266,275 | 31.38 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
7,221,477 | 100 | % | 7,221,477 | 100 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | Given the termination of the functions of Mr. Guy-Charles Fanneau de La Horie, previously President of the Management Board, on May 23, 2022, and of Mr. Philippe Rousseau, previously a member of the Management Board, on March 31, 2022, the Company’s shares that they held as of December 31, 2022 are not taken into account in this number of shares. This number corresponds to the shares held personally by Mr. Guy Rigaud, not included in the “GR Pool” line below, and by Mr. Didier Hoch, President of the Board of Directors. The shares held by Elaia Capital and Go Capital are indicated in the corresponding lines of the table. |
As of December 31, 2022 and to the best of the Company’s knowledge, no shareholder other than those mentioned in the table above held more than 5% of the Company’s capital and voting rights.
Each share gives the right to one vote. There is no limit to the number of votes that each shareholder may cast. There are no double voting rights.
| 6.2 | SECURITIES GIVING ACCESS TO THE SHARE CAPITAL |
The breakdown of the securities giving access to the Company’s capital and valid at December 31, 2022 are set out in the table below. In total, these securities give the right to subscribe to or issue 673,973 new shares, i.e., 8.53% of the capital existing at December 31, 2022.
27
Summary table of start-up stock options on December 31, 2022.
| Potential share capital |
Number | Parity | Maximum number of shares to be issued |
|||||||||
| 2017 start-up stock option |
— | 1 for 1 | — | |||||||||
| 2018 start-up stock option |
4,200 | 1 for 1 | 4,200 | |||||||||
| 2019 start-up stock option |
29,538 | 1 for 1 | 29,538 | |||||||||
| 2020 start-up stock option |
371,927 | 1 for 1 | 371,927 | |||||||||
| 2021 start-up stock option |
209,785 | 1 for 1 | 209,785 | |||||||||
| 2022 start-up stock option |
— | 1 for 1 | — | |||||||||
| Number of potential shares |
615,450 | |||||||||||
|
|
|
|||||||||||
| Number of existing shares |
7,221,477 | |||||||||||
|
|
|
|||||||||||
| Overall dilution |
7.854264 | % | ||||||||||
|
|
|
|||||||||||
Summary table of free shares on December 31, 2022.
| Date of allocation | Number of shares allocated |
In vesting period | In holding period |
|||||||||||||
| 2022 free share allocation |
May 19, 2022 | 58,523 | 58,523 | 0 | ||||||||||||
| 6.3 | CROSSING OF STATUTORY OWNERSHIP THRESHOLDS |
As the Company’s shares have been admitted to trading and listed since February 5, 2021, the provisions of Article L.233-7 of the French Commercial Code relating to the crossing of ownership thresholds have only been applicable since that date.
As of December 31, 2022, the Company had not received any ownership threshold crossing declarations pursuant to the provisions of Article L.233-7 of the French Commercial Code.
| 6.4 | EMPLOYEE SHAREHOLDING |
Below you will find the information required pursuant to Article L.225-102 of the French Commercial Code.
As of December 31, 2022, the Company’s employees held 0.007% of the Company’s capital on a non-diluted basis and 4.03% on a fully diluted basis.
We inform you that no company savings plan has been established for the benefit of the Company’s employees.
The Company did not acquire any shares during the fiscal year for allocation to employees, pursuant to Article L.225-208 of the French Commercial Code.
28
| 6.5 | INFORMATION ON TREASURY SHARES |
Following the admission to trading and the first listing of the Company’s shares on the Euronext Growth Paris market, the Company has entered into a liquidity contract with Portzamparc as of February 5, 2021 for a period of one year, renewable by tacit agreement.
This liquidity contract is executed within the framework of the share buyback program authorized by the fourteenth (14th) resolution of the Company’s Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020, and within the framework of the program authorized by the thirteenth (13th) resolution of the Company’s Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022.
This program has the following objectives, in order of priority:
| • | to promote the liquidity and boost the price of the Company’s securities on the Euronext Growth Paris market, or on any other market, through the intermediary of an investment service provider acting independently within the framework of a liquidity contract in compliance with the code of ethics recognized by the French Financial Markets Authority ( AMF); |
| • | to remit the repurchased securities in payment or exchange within the framework of a merger, spin-off or contribution; |
| • | to allocate shares to employees or corporate officers of the Company and of French or foreign companies or groupings affiliated with it, in accordance with legal and regulatory conditions, in particular within the framework of profit-sharing schemes, employee shareholding plans or company savings plans, stock option plans, or by means of free share allocations or any other condition permitted by regulations; |
| • | to allocate the shares repurchased upon the exercise of rights attached to securities giving the right, through redemption, conversion, exchange, presentation of a warrant or any other means, to existing shares to be issued by the Company; |
| • | where applicable, to allocate shares repurchased within the framework of the implementation of any market practice that may be accepted by the AMF and that complies with the regulations in force at the time of the effective buyback of the shares, it being specified that, in such case, the Company would inform its shareholders by means of a press release. |
The duration of the program is a maximum of 18 months from the ordinary general meeting of the shareholders of May 19, 2022. The program will expire either on the date on which any General Meeting of Shareholders of the Company adopts a new share buyback program or, failing that, on November 19, 2023.
The maximum authorized percentage of share capital that may be repurchased is 10%. The number of shares acquired by the Company with a view to their holding and subsequent remittance in payment or exchange within the framework of a merger, spin-off or contribution may not exceed 5% of its share capital.
The maximum unit purchase price is €20, i.e., a maximum theoretical amount devoted to the buyback program of €11,708,680.00 based on the maximum percentage of 10%, excluding trading fees.
29
This number of shares and the purchase price limits may be adjusted, where necessary, by the Board of Directors in the event of financial transactions by the Company or decisions affecting the share capital.
As of December 31, 2022, the following resources were included in the liquidity account:
| • | 22,057 Pherecydes Pharma shares; |
| • | €43,066.35. |
30
CHAPTER 7—TRANSACTIONS CARRIED OUT BY MANAGERS ON THEIR SHARES
Pursuant to the provisions of Articles 223-22 A and 223-26 of the AMF’s General Regulations, we hereby inform you that the following transactions on the Company’s securities were carried out by executive directors during the fiscal year:
None.
31
| CHAPTER | 8—COMPENSATION AND BENEFITS GRANTED TO CORPORATE OFFICERS AND SHAREHOLDING |
| 8.1 | COMPENSATION AND BENEFITS GRANTED TO CORPORATE OFFICERS |
The tables below show the compensation and benefits in kind paid to corporate officers by the Company during the fiscal years ended December 31, 2022 and December 31, 2021:
Summary table of all compensation, options and free shares granted to executive directors
A summary of all compensation due and paid to the members of the Board of Directors of the Company during the fiscal years ended December 31, 2022 and December 31, 2021 is presented below:
| Name |
2022 | 2021 | ||||||||||||||||||||||
| Fixed compensation due and paid (in euros) |
Variable compensation due and paid (in euros) |
Benefits in kind due and paid (in euros) |
Fixed compensation due and paid (in euros) |
Variable compensation due and paid (in euros) |
Benefits in kind due and paid (in euros) |
|||||||||||||||||||
| Guy-Charles Fanneau de La Horie President of the Management Board(1) |
99,764 | 97,389 | 2,307 | 235,200 | 37,632 | 5,538 | ||||||||||||||||||
| Philippe Rousseau Member of the Management Board(2) |
39,201 | 89,977 | 156,830 | 67,720 | — | |||||||||||||||||||
| Didier Hoch Chief Executive Officer(3) |
161,504 | |||||||||||||||||||||||
| Thibaut du Fayet Chief Executive Officer(4) |
131,249 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| TOTAL |
431,718 | 187,366 | 2,307 | 392,031 | 100,352 | 5,538 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | Mr. Guy-Charles Fanneau de La Horie was appointed member of the Management Board and President of the Management Board by the Supervisory Board at its meeting on December 1, 2015, from January 4, 2016 until May 23, 2022. |
| (2) | Mr. Philippe Rousseau was appointed member of the Management Board by the Supervisory Board at its meeting on June 24, 2020 and left the company on March 31, 2022. |
| (3) | Mr. Didier Hoch was appointed member of the Supervisory Board and President of the Supervisory Board on January 16, 2019; the Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022 changed the Company’s method of management and administration by adopting a Board of Directors and general management; Mr. Hoch was appointed Chief Executive Officer by the Board of Directors on May 23, 2022 until December 15, 2022. |
| (4) | Mr. Thibaut du Fayet was appointed Chief Operating Officer and member of the Management Board on April 7, 2022, and the Pharma General Meeting of Shareholders on May 19, 2022 approved the change in governance to a company with a Board of Directors. The new Board of Directors appointed Thibaut du Fayet as Chief Operating Officer as of May 23, 2022. On December 15, 2022, at the end of the Combined General Meeting of Shareholders on December 15, 2022, the Board of Directors appointed Mr. Thibaut du Fayet as Chief Executive Officer. |
32
Summary table of attendance fees and other compensation received by non-executive corporate officers
A summary of all attendance fees and other compensation paid to members of the Company’s Supervisory Board for the fiscal years ended December 31, 2021 and December 31, 2022 is presented below:
| NON-EXECUTIVE CORPORATE OFFICERS |
Fiscal year 2021 | Fiscal year 2022 | ||||||||||||||
| Amounts allocated |
Amounts paid |
Amounts allocated |
Amounts paid |
|||||||||||||
| Maryvonne Hiance, director (1) |
||||||||||||||||
| Compensation (fixed, variable) |
€ | 16,643 | € | 16,643 | € | 28,711 | € | 17,710 | ||||||||
| Other compensation |
— | — | — | — | ||||||||||||
| Elaia Partners, represented by Franck Lescure, director (2) |
||||||||||||||||
| Compensation (fixed, variable) |
€ | 0 | € | 0 | € | 0 | € | 0 | ||||||||
| Other compensation |
€ | 0 | € | 0 | € | 0 | € | 0 | ||||||||
| Go Capital, represented by Leila Nicolas, director (3) |
||||||||||||||||
| Compensation (fixed, variable) |
€ | 0 | € | 0 | € | 0 | € | 0 | ||||||||
| Other compensation |
€ | 0 | € | 0 | € | 0 | € | 0 | ||||||||
| Guy Rigaud, director (4) |
||||||||||||||||
| Compensation (fixed, variable) |
€ | 0 | € | 0 | € | 0 | € | 0 | ||||||||
| Other compensation |
€ | 0 | € | 0 | € | 0 | € | 0 | ||||||||
| Robert Sebbag, director (5) |
||||||||||||||||
| Compensation (fixed, variable) |
€ | 7.989 | € | 7.989 | € | 15,221 | € | 9,221 | ||||||||
| Other compensation |
€ | 0 | € | 0 | — | — | ||||||||||
| Eric Leire, director (6) |
||||||||||||||||
| Compensation (fixed, variable) |
€ | 10.794 | € | 10.794 | € | 19.026 | € | 11.526 | ||||||||
| Other compensation |
€ | 0 | € | 0 | — | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
€ | 96,887 | € | 96,887 | € | 62,598 | € | 38,458 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
33
| (1) | Ms. Maryvonne Hiance was appointed member of the Supervisory Board and President of the Supervisory Board on January 16, 2019; the Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022 appointed the current members of the Supervisory Board as the first directors. |
| (2) | Elaia Partners was appointed member of the Supervisory Board on May 28, 2019; the Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022 appointed the current members of the Supervisory Board as the first directors. |
| (3) | Go Capital was appointed member of the Supervisory Board on December 22, 2017; the Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022 appointed the current members of the Supervisory Board as the first directors. |
| (4) | Mr. Guy Rigaud was appointed member of the Supervisory Board on December 24, 2020; the Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022 appointed the current members of the Supervisory Board as the first directors. |
| (5) | Mr. Robert Sebbag was appointed member of the Supervisory Board on December 24, 2020; the Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022 appointed the current members of the Supervisory Board as the first directors. |
| (6) | Mr. Eric Leire was appointed member of the Supervisory Board on December 24, 2020; the Ordinary and Extraordinary General Meeting of Shareholders of May 19, 2022 appointed the current members of the Supervisory Board as the first directors. |
| 8.2 | SHAREHOLDINGS OF CORPORATE OFFICERS |
As of December 31, 2022, the shareholding of each officer in the share capital of the Company is as follows:
| Officers |
Shares | Voting rights | ||||||||||||||
| Number | % | Number | % | |||||||||||||
| Chief Executive Officer | ||||||||||||||||
| Thibaut du Fayet |
0 | 0 | % | 0 | 0 | % | ||||||||||
| Members of the Board of Directors | ||||||||||||||||
| Didier Hoch |
334 | N/S | 334 | N/S | ||||||||||||
| Maryvonne Hiance |
0 | 0 | % | 0 | 0 | % | ||||||||||
| Go Capital Represented by Ms. Leila Nicolas |
1,023,103 | 14.17 | % | 1,023,103 | 14.17 | % | ||||||||||
| Elaia Partners Represented by Mr. Franck Lescure |
1,491,037 | 20.65 | % | 1,491,037 | 20.65 | % | ||||||||||
| Guy Rigaud |
3,997 | 0.05 | % | 3,997 | 0.05 | % | ||||||||||
| Eric Leire |
0 | 0 | % | 0 | 0 | % | ||||||||||
| Robert Sebbag |
0 | 0 | % | 0 | 0 | % | ||||||||||
34
CHAPTER 9—SPECIAL REPORT ON STOCK OPTIONS AND FREE SHARES
Dear Shareholders,
This report is presented to you:
| • | pursuant to the provisions of Article L.225-184 of the French Commercial Code concerning transactions relating to share subscription or purchase options, and |
| • | pursuant to the provisions of Article L.225-197-4 of the French Commercial Code concerning transactions relating to free shares. |
| 9.1 | SHARE SUBSCRIPTION OR PURCHASE OPTIONS |
Allocation of share subscription options during the fiscal year ended December 31, 2022
No share subscription or purchase options were allocated during the fiscal year ended December 31, 2022.
Exercise by beneficiaries of share subscription options during the fiscal year ended December 31, 2022
No share subscription or purchase options were exercised during the fiscal year ended December 31, 2022.
| 9.2 | FREE SHARE ALLOCATIONS |
Free share allocation during the fiscal year ended December 31, 2022
The ordinary and extraordinary general meeting of the shareholders of May 19, 2022, in its twentieth (20th) resolution, authorized the Board of Directors, in accordance with the provisions of Articles L.225-197-1 et seq. of the French Commercial Code, to proceed with a free allocation of the Company’s shares, either existing or to be issued, on one or more occasions, to the staff members that it determines from among the eligible corporate officers and employees of the Company and of companies or groupings affiliated with it under the terms of Article L.225-197-2 of said code.
Under this authorization, the Board of Directors unanimously decided at its meeting on May 19, 2022 to allocate a total of 58,523 free shares to be issued by the Company.
| Plan |
Number of shares allocated |
Vesting date |
Transferability date | |||
| May 19, 2022 |
58,523 | 25% of the free share allocations will vest on May 19, 2023; 25% of the free share allocations will vest on May 19, 2024; 25% of the free share allocations will vest on May 19, 2025; The balance will vest on May 19, 2026. |
One year from the expiration of the vesting period of each tranche. |
35
Free shares granted to each of the corporate officers
| Beneficiaries |
Number of shares allocated |
Date of allocation |
Vesting period | Holding period | ||||||||||||
| Thibaut du Fayet |
58,523 | May 19, 2022 | 1 year | 1 year | ||||||||||||
36
Vesting of free shares during the fiscal year ended December 31, 2022
During the fiscal year ended December 31, 2022, the Board of Directors of the Company noted the acquisition by Mr. Guy-Charles Fanneau de La Horie of 2,688 free shares and by Mr. Philippe Rousseau of 4,480 free shares, all of which were allocated on February 4, 2021.
The holding period for all these free shares vested during the fiscal year ended December 31, 2022 ended on February 4, 2023.
37
CHAPTER 10—APPOINTMENT OF THE STATUTORY AUDITORS
| 10.1 | STATUTORY AUDITORS |
Primary statutory auditors
PricewaterhouseCoopers Audit
Represented by Mr. Cédric Mazille
63, Rue de Villiers
92208 Neuilly-sur-Seine, France
Appointed by the initial Articles of Association of December 12, 2006 and reappointed by the Ordinary and Extraordinary General Meeting of Shareholders of May 28, 2020 for a term of six fiscal years, i.e. until the end of the General Meeting of Shareholders called in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
Alternate statutory auditor
Mr. Patrice Morot
63, Rue de Villiers
92208 Neuilly-sur-Seine, France
Appointed by the Ordinary and Extraordinary General Meeting of Shareholders of May 28, 2020 for a term of six fiscal years, i.e. until the end of the General Meeting of Shareholders called in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
| 10.2 | STATUTORY AUDITORS WHO RESIGNED, WERE DISMISSED OR WERE NOT REAPPOINTED DURING THE FISCAL YEAR ENDED DECEMBER 31, 2022 |
None.
38
CHAPTER 11—LIST OF RELATED-PARTY AGREEMENTS AND CURRENT AGREEMENTS
Pursuant to the provisions of Articles L. 225-38 et seq. of the French Commercial Code, below you will find a list of related-party agreements and current agreements for the fiscal year ended December 31, 2022.
We hereby inform you that the functions of the individuals concerned by the agreements described in this chapter are considered as of the date of signature of said agreements.
To the best of the Company’s knowledge, there are no service contracts between the Company and its corporate officers providing for the granting of benefits other than those described in this chapter.
| 11.1 | NEW RELATED-PARTY AGREEMENTS ENTERED INTO DURING FISCAL YEAR 2022 |
None.
| 11.2 | NEW RELATED-PARTY AGREEMENTS ENTERED INTO SINCE THE END OF FISCAL YEAR 2022 |
None.
| 11.3 | RELATED-PARTY AGREEMENTS APPROVED BY THE GENERAL MEETING OF SHAREHOLDERS, BUT WHICH CONTINUE TO BE IN EFFECT DURING FISCAL YEAR 2022 |
None.
| 11.4 | SURETIES, ENDORSEMENTS AND GUARANTEES GIVEN BY THE COMPANY TO THIRD PARTIES |
None.
| 11.5 | AGREEMENTS BETWEEN A CORPORATE OFFICER OR A SHAREHOLDER HOLDING MORE THAN 10% OF THE VOTING RIGHTS OF THE COMPANY AND A SUBSIDIARY, EXCLUDING CURRENT AGREEMENTS |
None.
39
CHAPTER 12—OTHER INFORMATION
In accordance with the provisions of Articles 39-4 and 223 quater of the French General Tax Code, we hereby inform you that the Company’s financial statements for the past year do not contain any expenses that are not deductible from taxable income.
Pursuant to the provisions of Article L.232-6 of the French Commercial Code, we hereby inform you that no changes have been made to the method of presentation of the annual financial statements, nor to the valuation methods used in comparison with the previous fiscal year.
40
CHAPTER 13—REPORT OF THE BOARD OF DIRECTORS
In accordance with the provisions of Article L.225-68 paragraph 6 of the French Commercial Code, in Appendix 1 of this management report you will find the report on corporate governance prepared by the Board of Directors of the Company pursuant to the aforementioned provisions.
We hope that the above will meet with your approval.
Nantes, France,
March 30, 2023
Chief Executive Officer
Thibaut du Fayet
41
PHERECYDES PHARMA
Limited company (société anonyme) with a Board of Directors and capital of €7,939,179
Registered office: 22, Boulevard Benoni Goullin – 44200 Nantes, France
493 252 266 Nantes Trade and Companies Register
REPORT OF THE BOARD OF DIRECTORS ON CORPORATE GOVERNANCE
Dear Shareholders,
This report is hereby presented to you pursuant to Articles L. 225-37 et seq. and L. 22-10-8 et seq. of the French Commercial Code and concerns corporate governance, as well as the observations of the Board of Directors of Pherecydes Pharma (hereinafter the “Company”) and the financial statements for the fiscal year ended December 31, 2022.
We hereby remind you that the Company’s shares are admitted to trading on the Euronext Growth Paris market, an organized multilateral trading facility. As a result, in accordance with the provisions of Article L.225-37 of the French Commercial Code, this report includes all the information required by Articles L.225-37 et seq. and L.22-10-8 et seq. of said Code for limited companies (sociétés anonymes) whose securities are not listed on a regulated market.
CHAPTER 14—REPORT ON CORPORATE GOVERNANCE
| 14.1 | LIST OF OFFICES |
| 14.1.1 | Chief Executive Officer |
As of December 31, 2022, the Company has a Chief Executive Officer, Mr. Thibaut du Fayet (55 years old). He was unanimously appointed Chief Executive Officer by the Board of Directors of the Company at its meeting of December 15, 2022 for an indefinite term.
Mr. Thibaut du Fayet, Chief Executive Officer (55 years old)
Thibaut du Fayet brings to the Company over 20 years of experience in the pharmaceutical and life sciences industry. After obtaining an MBA from ESSEC Business School in 1992, he was Senior Managing Director at Bossard-Gemini for six years. From 2000, he held management positions first at Rhône Poulenc as Strategy Director for four years, and then at BioMerieux where he was also Strategic Marketing Director for four years. In 2008, he joined Transgene where he held the position of VP Corporate Development, Alliances & Programs Management for 13 years.
Through his various leadership roles in development, strategy, business development, and program management, he has acquired important and diverse skills in the pharmaceutical industry.
42
Mr. Thibaut du Fayet was appointed a member of the former Management Board by the former Supervisory Board at its meeting of April 5, 2022, to replace Mr. Philippe Rousseau for a term of four (4) years, i.e., until the end of the General Meeting of Shareholders called in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. The meeting therefore dismissed all the members of its Management Board, including Mr. Thibaut du Fayet.
On May 23, 2022, the Board of Directors of the Company unanimously appointed Mr. Thibaut du Fayet as Chief Operating Officer of the Company for an indefinite term. On December 15, 2022, the Board of Directors of the Company unanimously appointed Mr. Thibaut du Fayet as Chief Executive Officer of the Company for an indefinite term.
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| None |
Other offices and positions held over the last five years
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Chief Operating Officer | Pherecydes Pharma | 2022 | ||
| Member of the Management Board | Pherecydes Pharma | 2022 | ||
| Outside the Group | ||||
| None | ||||
| Number of shares held as of December 31, 2022: 0 | ||||
| 14.1.2 | BOARD OF DIRECTORS |
Composition of the Board of Directors
As of December 31, 2022, the Board of Directors of the Company is composed as follows:
| Name |
Office |
Age |
Independent |
Member of |
Member of the |
Date of |
Expiration | |||||||
| Didier Hoch |
President of the Board of Directors |
66 | 2022 | 2026 | ||||||||||
| Maryvonne Hiance |
Director | 74 | ✓ | 2022 | 2026 |
43
| Name |
Office |
Age |
Independent |
Member of |
Member of the |
Date of |
Expiration | |||||||
| Go Capital – represented by Leila Nicolas |
Director | 42 | ✓ | 2022 | 2026 | |||||||||
| Elaia Partners – represented by Franck Lescure |
Director | 54 | 2022 | 2026 | ||||||||||
| Guy Rigaud |
Director | 75 | ✓ | 2022 | 2026 | |||||||||
| Robert Sebbag |
Director | 72 | ✓ | 2022 | 2026 | |||||||||
| Eric Leire |
Director | 65 | ✓ | ✓ | 2022 | 2026 |
| (1) | In accordance with recommendation no. 9 of the Middlenext corporate governance code for small and mid-sized companies of September 2021. |
Profiles of the members of the Board of Directors
Didier Hoch, President of the Board of Directors (66 years old)
Didier Hoch, M.D. is a director of a listed company, OSE Immunotherapeutics, and was previously a member of the board of DBV Technologies and Genticel. From 2011 to 2013, he was President of Pevion Biotech, and from 2013 to 2018, President of the Biovision Forum and of the startup incubator “Big Booster.” From 2000 to 2010, he was President of Sanofi-Pasteur-MSD, a joint venture company between Sanofi & Merck dedicated to vaccines. Didier Hoch also held various managerial positions at Rhône Poulenc Rorer, then Aventis (“VP Middle East-Africa”). He was also President of the vaccine manufacturers association “Vaccine Europe” and President of the Biotechnology Committee of LEEM (Les Entreprises du médicament).
Mr. Didier Hoch was co-opted as a member of the former Supervisory Board by the Supervisory Board at its meeting of January 16, 2019, to replace Mr. Michel Joli for the remainder of the latter’s term of office, i.e., until the end of the General Meeting of Shareholders called in 2022 to approve the financial statements for the fiscal year ended December 31, 2021. The Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 decided to reduce the term of office of members of the Supervisory Board from six to four years. As a result, the Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 decided to renew his term of office of member of the Supervisory Board for a period of four years, i.e. until the end of the General Meeting of Shareholders called in 2024 to approve the financial statements for the fiscal year ended December 31, 2023.
44
The former Supervisory Board, at its meeting on January 16, 2019, decided to appoint Mr. Didier Hoch as President of the Supervisory Board for the duration of his term of office of member of the Supervisory Board, and he was reappointed by the Supervisory Board at its meeting on December 24, 2020.
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. As a result, the meeting dismissed all the members of its Supervisory Board, including Mr. Didier Hoch, whose terms of office of both member of the Supervisory Board and of President of the Supervisory Board were terminated.
In addition, the same meeting of May 19, 2022 appointed Mr. Didier Hoch as a director of the Company for a term of four (4) years, i.e. until the end of the General Meeting of Shareholders to be held in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
On May 19, 2022, the directors of the Company unanimously appointed Mr. Didier Hoch as President of the Board of Directors for the duration of his term of office as a member of the Board of Directors. Mr. Didier Hoch was unanimously appointed Chief Executive Officer by the Board of Directors on May 23, 2022. He held the position of Chief Executive Officer until December 15, 2022.
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| Director | Ose Immuno Therapeutics | 2011 | ||
| Co-President | Charity for the Underground University Foundation | 2018 | ||
| President of the Strategic Board | Goliver Therapeutics | 2019 |
Other offices and positions held over the last five years
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Chief Executive Officer | Pherecydes Pharma | 2022 | ||
| President of the Supervisory Board | Pherecydes Pharma | 2019–2022 | ||
| Outside the Group | ||||
| Director | Germitec | 2014–2019 | ||
| Director | DBV Technologies | 2012–2016 | ||
| Director | Genticel | 2011–2017 | ||
| Advisory Scientific Board member | Myasterix/Curavac | 2014–2018 | ||
| President of Biovision and Big Booster | Fondation pour l’Université de Lyon | 2012–2018 | ||
| Number of shares held as of December 31, 2022: 334 | ||||
45
Maryvonne Hiance, member of the Board of Directors (74 years old)
Previously President and co-founder of Effimune, Maryvonne Hiance is a nuclear engineer who managed a nuclear neutron program at FRAMATOME (Areva) for 14 years. She also previously held managerial positions at various innovative companies in Biotechnology for more than 20 years: SangStat Atlantic (the parent company Sangstat medical corporation was acquired by industrialist Genzyme in 2003 for its portfolio of products in immune suppression and transplantation). She also managed the innovative companies DrugAbuse Sciences and TcLand. Maryvonne Hiance founded and led Strategic Ventures, a consulting firm involved in helping technology companies. She was a member of the Strategic Innovation Council and advisor to the French Minister of SMEs and Industry. She is currently Vice-President of France Biotech.
Ms. Maryvonne Hiance was co-opted as a member of the former Supervisory Board by the Supervisory Board at its meeting of January 16, 2019, to replace Mr. Jean-Pierre Loza for the remainder of the latter’s term of office, i.e., until the end of the General Meeting of Shareholders called in 2019 to approve the financial statements for the fiscal year ended December 31, 2018. The Ordinary and Extraordinary General Meeting of Shareholders of June 28, 2019 resolved to reappoint Ms. Maryvonne Hiance as a member of the Board of Directors for a period of six years. The Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 decided to reduce the term of office of members of the Supervisory Board from six to four years and noted, as a result, that the term of office of Ms. Maryvonne Hiance as a member of the Supervisory Board will expire at the end of the General Meeting of Shareholders called in 2023 to approve the financial statements for the fiscal year ended December 31, 2022.
The former Supervisory Board, at its meeting on January 16, 2019, decided to appoint Ms. Maryvonne Hiance as Vice-President of the Supervisory Board for the duration of her term of office of member of the Supervisory Board.
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. As a result, the meeting dismissed all the members of its Supervisory Board, including Ms. Maryvonne Hiance, whose terms of office of both member of the Supervisory Board and of Vice-President of the Supervisory Board were terminated.
In addition, the same meeting of May 19, 2022 appointed Ms. Maryvonne Hiance as a director of the Company for a term of four (4) years, i.e. until the end of the General Meeting of Shareholders to be held in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| Vice-Chairwoman, strategy director | Ose Immuno Therapeutics | 2016 | ||
| Vice-President | France Biotech | 2019 | ||
| President of HealthTECH For Care | France Biotech | 2019 | ||
| President | Olgram | 2020 |
46
Other offices and positions held over the last five years
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Vice-President of the Supervisory Board | Pherecydes Pharma | 2019–2022 | ||
| Outside the Group | ||||
| President | France Biotech | 2016–2019 | ||
| Director | Apave | 2018–2020 | ||
| Number of shares held as of December 31, 2022: 0 | ||||
Mr. Franck Lescure, permanent representative of Elaia Partners, member of the Board of Directors (54 years old)
Franck Lescure joined Elaia Partners to bring his expertise in life sciences investment after more than 20 years of experience in early-stage biotech startups. Franck Lescure began his career as a scientist at Genset, one of the first French biotech startups. In 1997, he joined the Health subsidiary of the Air Liquide Group as a product manager. He started his career as an investor at Crédit Lyonnais Private Equity and then became a Partner in Life Sciences at Auriga Partners.
The company Elaia Partners was co-opted as a member of the former Supervisory Board by the Supervisory Board at its meeting of May 28, 2019, to replace the company Auriga Partners for the remainder of the latter’s term of office, i.e., until the end of the General Meeting of Shareholders called in 2021 to approve the financial statements for the fiscal year ended December 31, 2020. The Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 decided to reduce the term of office of members of the Supervisory Board from six to four years. As a result, the Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 decided to renew its term of office of member of the Supervisory Board for a period of four years, i.e. until the end of the General Meeting of Shareholders called in 2024 to approve the financial statements for the fiscal year ended December 31, 2023. Mr. Franck Lescure has been the permanent representative of Elaia Partners on the Company’s Supervisory Board since its co-option on May 28, 2019.
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. The meeting therefore dismissed all the members of its Supervisory Board, including the company Elaia Partners.
In addition, the same meeting of May 19, 2022 appointed the company Elaia Partners as a director of the Company for a term of four (4) years, i.e. until the end of the General Meeting of Shareholders to be held in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
Mr. Franck Lescure has been the permanent representative of Elaia Partners on the Board of Directors of the Company since its appointment on May 19, 2022.
47
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| Permanent representative of Elaia Partners | Flash Therapeutics | 2016 | ||
| Permanent representative of Elaia Partners | Honing Biosciences | 2020 | ||
| Permanent representative of Elaia Partners | Pili | 2019 | ||
| Permanent representative of Elaia Partners | Pylote | 2015 | ||
| Permanent representative of Elaia Partners | Aviwell | 2021 |
Other offices and positions held over the last five years
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Member of the Supervisory Board | Pherecydes Pharma | 2019–2022 | ||
| Outside the Group | ||||
| Permanent representative of Elaia Partners | Amoeba | 2014–2018 | ||
| Permanent representative of Elaia Partners | Cytoo | 2009–2018 | ||
| Permanent representative of Elaia Partners | Median | 2008–2018 | ||
| Permanent representative of Elaia Partners | Nosopharm | 2016–2019 | ||
| Permanent representative of Elaia Partners | Fab’entech | 2014–2019 | ||
| Permanent representative of Elaia Partners | Exeliom | 2019 | ||
| Permanent representative of Elaia Partners | EnobraQ | 2017–2020 | ||
| Number of shares held as of December 31, 2022: 0(1) | ||||
| (1) | As of the date of this report on corporate governance and to the best of the Company’s knowledge, Mr. Franck Lescure does not directly or indirectly hold any shares in the Company. The participation in the funds managed by Elaia Partners is described in chapter 8 of the management report. |
Ms. Leïla Nicolas, permanent representative of Go Capital, member of the Board of Directors (42 years old)
Leïla Nicolas started her career in 2004 as a product manager at Bayer Schering Pharma in the field of multiple sclerosis and oncology. She then joined the Biotechnology and Life Sciences sector as strategic marketing manager of a start-up in Alsace (Polyplus-transfection). Leïla Nicolas concluded several licensing agreements and acquired a strong expertise in intellectual property. Leïla Nicolas was then involved in the creation of a company that develops and sells environmental health analysis kits before joining Go Capital in late 2013.
The company Go Capital was appointed as a member of the former Supervisory Board by the Ordinary and Extraordinary General Meeting of Shareholders of December 22, 2017 for a term of six years. The Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 decided to reduce the term of office of members of the Supervisory Board from six to four years and noted, as a result, that the term of office of Go Capital as a member of the Supervisory Board will expire at the end of the General Meeting of Shareholders called in 2021 to approve the financial statements for the fiscal year ended December 31, 2020. The Ordinary General Meeting of Shareholders of June 22, 2021 renewed Go Capital’s term of office of member of the Supervisory Board for a period of four years, i.e. until the end of the General Meeting of Shareholders called in 2025 to approve the financial statements for the fiscal year ended December 31, 2024. Ms. Leila Nicolas has been the permanent representative of Go Capital on the Company’s Supervisory Board since its appointment on December 22, 2017.
48
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. The meeting therefore dismissed all the members of its Supervisory Board, including the company Go Capital.
In addition, the same meeting of May 19, 2022 appointed the company Go Capital as a director of the Company for a term of four (4) years, i.e. until the end of the General Meeting of Shareholders to be held in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
Ms. Leila Nicolas has been the permanent representative of Elaia Partners on the Board of Directors of the Company since its appointment on May 19, 2022.
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| Member of the Strategy Committee | HTC Assistance | 2021 | ||
| Member of the Strategy Committee | Kalsiom | 2020 | ||
| Director | Acticor Biotech | 2020 | ||
| Director | VitaDx | 2017 | ||
| Director | Coave Therapeutics (formerly Horama) | 2016 | ||
| Member of the Strategy Committee | Tricares | 2015 | ||
| Member of the Strategy Committee | I-SEP | 2015 |
Other offices and positions held over the last five years
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Member of the Supervisory Board | Pherecydes Pharma | 2017–2022 | ||
| Outside the Group | ||||
| Member of the Strategy Committee | Atlanthera | 2014–2022 | ||
| Member of the Strategy Committee | Biosency | 2018–2022 | ||
| Director | Graftys | 2019–2020 | ||
| Member of the Strategy Committee | Carlina | 2014–2020 | ||
| Member of the Strategy Committee | Kemiwatt | 2014–2018 | ||
| Member of the Strategy Committee | Surfact’Green | 2016–2018 | ||
| Director | VitamFero | 2014–2018 | ||
| Member of the Strategy Committee | Biosency | 2018–2021 | ||
| Member of the Strategy Committee | Celenys | 2014–2017 | ||
| Number of shares held as of December 31, 2022: 0(1) | ||||
| (1) | As of the date of this report on corporate governance and to the best of the Company’s knowledge, Ms. Leïla Nicolas does not directly or indirectly hold any shares in the Company. The participation in the funds managed by Go Capital is described in chapter 8 of the management report. |
49
Mr. Guy Rigaud, member of the Board of Directors (75 years old)
Guy Rigaud has 30 years of experience in private equity in more than 300 young regional companies (more than half of them in the technology sector). Founder and President of the Management Board of Rhône Alpes Création from 1990 to 2012, Guy Rigaud has participated in five IPOs on Euronext Paris and two on Nasdaq (in the context of industrial disposals). Guy Rigaud was a member of the Board of Directors of April Group (insurance), a company listed on Euronext Paris, for 12 years. Since 2012, Guy Rigaud is the founder and associate director of a seed capital fund created with four “family offices.”
Mr. Guy Rigaud was appointed as non-voting member of the former Supervisory Board by the Ordinary and Extraordinary General Meeting of Shareholders of December 22, 2017 for a term of six years. The Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 noted the resignation of Mr. Guy Rigaud from his position as non-voting member and resolved to appoint him as a member of the former Supervisory Board for a term of four years, i.e., until the end of the General Meeting of Shareholders called in 2024 to approve the financial statements for the fiscal year ended December 31, 2023.
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. The meeting therefore dismissed all the members of its Supervisory Board, including Mr. Guy Rigaud.
In addition, the same meeting of May 19, 2022 appointed Mr. Guy Rigaud as a director of the Company for a term of four (4) years, i.e. until the end of the General Meeting of Shareholders to be held in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| None | ||||
| Other offices and positions held over the last five years | ||||
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Member of the Supervisory Board | Pherecydes Pharma | 2017–2022 | ||
| Outside the Group | ||||
| Permanent representative of Eurekap | Kalray | |||
| Member of the Board of Directors | Kreaxi | |||
| Member of the Strategy Committee | LX Repair | |||
| Member of the Strategy Committee | GlycoBar | |||
| Number of shares held as of December 31, 2022: 3,997 | ||||
Mr. Robert Sebbag, member of the Board of Directors (72 years old)
Robert Sebbag, M.D. is attached to Public Hospitals of Paris (Hôpitaux de Paris) and works at the Pitié Salpêtrière hospital in the department of infectious and tropical diseases. He is also a member of the board and founding member of Action Contre la Faim and member of the board of Fondation Mérieux.
50
From 2006 to 2016, he was Vice President Access to Medicines at Sanofi. He previously held several positions at Rhône Poulenc Santé, Fondation Elf, and Aventis-Pasteur. He is a former member of the Executive Committee of the CEO round table of the Gate Foundation and a member of the board of LEEM (Les Entreprises du médicament).
Mr. Robert Sebbag was appointed as a member of the former Supervisory Board by the Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 for a term of four years, i.e. until the end of the General Meeting of Shareholders called in 2024 to approve the financial statements for the fiscal year ended December 31, 2023.
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. The meeting therefore dismissed all the members of its Supervisory Board, including Mr. Robert Sebbag.
In addition, the same meeting of May 19, 2022 appointed Mr. Robert Sebbag as a director of the Company for a term of four (4) years, i.e. until the end of the General Meeting of Shareholders to be held in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| Fondation Merieux | ||||
| Action contre la faim | ||||
| Provepharm | ||||
Other offices and positions held over the last five years
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Member of the Supervisory Board | Pherecydes Pharma | 2020–2022 | ||
| Outside the Group | ||||
| Positive Planet | ||||
| Number of shares held as of December 31, 2022: 0 | ||||
Mr. Eric Leire, member of the Board of Directors (65 years old)
Eric Leire, M.D. is currently CEO of Genflow Biosciences. He is also a board member of publicly traded and private biotechnology companies (Immunethep, BSIM Therapeutics and Inhatarget Therapeutics). Dr. Leire has served as CEO of several private and publicly traded U.S. biotechnology companies. He has listed several biotech companies on various stock exchanges (OMX.Nasdaq; OTC.QB and Nasdaq). He was a research associate at the Harvard AIDS Institute and Partner at Biofund Venture Capital. He has an M.D. from the University of Grenoble and a MBA from HEC and Kellogg School of Management, Northwestern University. He is also the inventor of numerous patents in the pharmaceutical field.
51
Mr. Eric Leire was appointed as a member of the former Supervisory Board by the Ordinary and Extraordinary General Meeting of Shareholders of December 24, 2020 for a term of four years, i.e. until the end of the General Meeting of Shareholders called in 2024 to approve the financial statements for the fiscal year ended December 31, 2023.
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management. The meeting therefore dismissed all the members of its Supervisory Board, including Mr. Eric Leire.
In addition, the same meeting of May 19, 2022 appointed Mr. Eric Leire as a director of the Company for a term of four (4) years, i.e. until the end of the General Meeting of Shareholders to be held in 2026 to approve the financial statements for the fiscal year ended December 31, 2025.
Other offices and positions held as of December 31, 2022
| Positions |
Companies |
Start dates | ||
| CEO | Genflow Biosciences | 2010 | ||
| President | Immunethep | 2018 | ||
| Non-executive director | BSIM | 2018 | ||
| Non-executive director | Inhatarget | 2020 |
Other offices and positions held over the last five years
| Positions |
Companies |
Dates | ||
| Within the Company | ||||
| Member of the Supervisory Board | Pherecydes Pharma | 2020–2022 | ||
| Outside the Group | ||||
| None | ||||
| Number of shares held as of December 31, 2022: 0 | ||||
| 14.1.3 | Censor |
Pursuant to Article 17 of the articles of association, the Board of Directors may appoint non-voting members. These non-voting members, the number of which may not exceed three (3), form an advisory board. They are freely chosen because of their skills and expertise.
They are appointed for a term of four (4) years expiring at the end of the Ordinary General Meeting of Shareholders called to approve the financial statements for the previous fiscal year and held in the year during which the term of office of said non-voting member expires.
The board of non-voting members examines the questions that the Board of Directors or its President submits to it for review. The non-voting members attend the meetings of the Board of Directors and take part in the deliberations in an advisory capacity only, but their absence does not affect the validity of the deliberations.
They are called to the meetings of the Board of Directors under the same conditions as the voting members. The Board of Directors may compensate the non-voting members by deducting their compensation from the total compensation allocated by the General Meeting of Shareholders to the members of the Board of Directors.
52
As of December 31, 2022, the Company has no non-voting members of the Board of Directors.
| 14.1.4 | Change in the composition of the former Management Board, the former Supervisory Board, and the Board of Directors since January 1, 2022 |
Change in the composition of the former Supervisory Board since January 1, 2022
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management and, as a result, dismissed all the members of its Supervisory Board.
Change in the composition of the former Management Board since January 1, 2022
On May 19, 2022, the Ordinary General Meeting of the Company’s Shareholders resolved to adopt a method of management and administration with a Board of Directors and general management and, as a result, dismissed all the members of its Management Board.
Change in the composition of the Board of Directors since January 1, 2022
The Ordinary General Meeting of the Company’s Shareholders held on May 19, 2022 established the Board of Directors by appointing as directors:
| • | Mr. Didier Hoch; |
| • | Ms. Maryvonne Hiance; |
| • | Mr. Guy Rigaud; |
| • | Mr. Robert Sebbag; |
| • | Mr. Eric Leire; |
| • | The company Go Capital; and |
| • | The company Elaia Partners. |
On May 19, 2022, the Board of Directors of the Company unanimously appointed Mr. Didier Hoch as President of the Board of Directors for the duration of his term of office as a member of the Board of Directors.
Since May 19, 2022, the Board of Directors of the Company has not changed.
Change in the composition of non-voting members of the Board of Directors since January 1, 2022
None.
53
| 14.2 | DELEGATIONS OF AUTHORITY FOR CAPITAL INCREASES DURING THE FISCAL YEAR ENDED DECEMBER 31, 2022 |
The table below summarizes the delegations of authority granted to the Board of Directors by the ordinary and extraordinary general meeting of the shareholders of the Company held on May 19, 2022 (hereinafter, the “CGM”) and by the Extraordinary General Meeting of Shareholders of the Company held on December 15, 2022 (hereinafter, the “EGM”). Please note that the minutes of the EGM and the CGM will soon be available on the Company’s website in the “Shareholders” section (www.pherecydes-pharma.com/).
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 1. Issue of ordinary shares or other securities giving access to the share capital of the Company with preferential subscription rights for Shareholders (fifteenth resolution of the CGM of May 19, 2022). |
€4,000,000 | Delegation of authority expired following the implementation of a new delegation of authority by the EGM of December 15, 2022. | None. | The Board of Directors has full powers, including the right to sub-delegate such powers in accordance with the law, to determine, under the conditions laid down by the law and the articles of association, the dates, terms and conditions of any issues, as well as the form and characteristics of the shares or securities giving access to the share capital or debt securities to be issued, with or without premium. |
54
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 2. Issue of ordinary shares or other securities giving access to the share capital of the Company, without the preferential subscription rights of shareholders, by way of a public offering other than those referred to in Article L.411-2-1° of the French Monetary and Financial Code (sixteenth resolution of the CGM of May 19, 2022)(1). |
€4,000,000 | Delegation of authority expired following the implementation of a new delegation of authority by the EGM of December 15, 2022. | €215,943/215,943 shares (September 21, 2022). | The amount to be received by the Company for each of the shares issued or to be issued under this delegation of authority, after taking into account, in the event of the issue of autonomous share subscription or allocation warrants, the issue price of said warrants, will be set by the Board of Directors and must be at least equal to the average weighted by the Company share price volumes on the Euronext Growth Paris market over the last five (5) trading sessions preceding the pricing of the issue, potentially reduced by a maximum discount of 25%. |
55
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 3. Issue of ordinary shares or other securities giving access to the share capital of the Company, without the preferential subscription rights of shareholders, up to a maximum of 20% of the share capital per year, in the context of public offerings aimed exclusively at a limited circle of investors acting on their own behalf or at the qualified investors referred to in Article L.411-2-1° of the French Monetary and Financial Code (seventeenth resolution of the CGM of May 19, 2022)(2). |
€4,000,000 | Delegation of authority expired following the implementation of a new delegation of authority by the EGM of December 15, 2022. | €345,406/345,406 shares (September 21, 2022). | The amount received or to be received by the Company for each of the shares issued or to be issued under this delegation of authority, after taking into account, in the event of the issue of autonomous share subscription or allocation warrants, the issue price of said warrants, will be set by the Board of Directors and must be at least equal to the average weighted by the Company share price volumes on the Euronext Growth Paris market over the last five (5) trading sessions preceding the pricing of the issue, potentially reduced by a maximum discount of 25%. |
56
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 4. Issue of ordinary shares or other securities giving access to the share capital of the Company to designated categories of investors (eighteenth resolution of the CGM of May 19, 2022)(3). |
€4,000,000 | Delegation of authority expired following the implementation of a new delegation of authority by the EGM of December 15, 2022. | €759,481/759,481 shares (September 21, 2022). | The subscription price of such securities and their dividend entitlement date will be determined by the board of directors, it being specified that the amount received or to be received by the Company for each of the shares issued under this delegation of authority will be at least equal to the average weighted by the Company share price volumes on the Euronext Growth Paris market over the last five (5) trading sessions preceding the pricing of the issue. This average may be corrected to take into account differences in the dividend entitlement date and may be reduced by a maximum discount of 25%. |
57
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount of the capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 5. Free allocation of new or existing shares of the company (twentieth resolution of the CGM of May 19, 2022)(4). |
Free share allocations carried out under this authorization may not give entitlement to a total number of shares exceeding 10% of the number of shares comprising the share capital calculated at the date of allocation. | July 19, 2025. | 58,523 shares (May 19, 2022). | Free. |
58
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount of the capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 6. Allocation of Company share subscription or purchase options (twenty-first resolution of the CGM of May 19, 2022)(5). |
Share subscription and purchase options granted under this authorization may not give entitlement to a total number of shares exceeding 10% of the number of shares comprising the share capital calculated at the date of allocation. | July 19, 2025. | None. | The exercise price of the options will be set by the Board of Directors on the day the options are granted and may not be less than (a) in the case of subscription options, the lower between 80% of the average of the prices quoted on the five trading sessions preceding the day on which the options are granted and the subscription price of the most recent capital increase preceding the day on which the options are granted, and (b) in the case of purchase options, the value indicated in (a) above, or the average purchase price of the shares referred to in Article L.225-179 of the French Commercial Code. |
59
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount of the capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 7. Issue of share subscription warrants, without preferential subscription rights, to a category of persons (twenty-second resolution of the CGM of May 19, 2022). |
The exercise of the warrants issued under this resolution may not give rise to a number of ordinary shares representing more than 15% of the number of shares comprising the share capital calculated at the date of allocation. | November 19, 2023. | None. | The Board of Directors shall have full powers, with the option to sub-delegate such powers under legal and regulatory conditions, to determine the subscription and exercise conditions of the share subscription warrants, and in particular the time frames and dates of exercise of the share subscription warrants, the number of shares to which each share subscription warrant entitles the holder, the terms and conditions for paying up the shares subscribed for by exercising the share subscription warrants, as well as their dividend entitlement date, which may be retroactive. |
60
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 8. Issue of ordinary shares or other securities giving access to the share capital of the Company with preferential subscription rights for shareholders (second resolution of the EGM of December 15, 2022). |
€4,000,000 | February 15, 2025 | None. | The Board of Directors has full powers, including the right to sub-delegate such powers in accordance with the law, to determine, under the conditions laid down by the law and the articles of association, the amount of the capital increase and, more generally, the amount of the issue in the event of the issue of securities, the issue price and the amount of the premium which may, where applicable, be requested on issue. |
61
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 9. Issue of ordinary shares or other securities giving access to the share capital of the Company, without the preferential subscription rights of shareholders, by way of a public offering, excluding those referred to in Article L.411-2-1° of the French Monetary and Financial Code (third resolution of the EGM of December 15, 2022). |
€4,000,000 | February 15, 2025 | None. | The issue price of the shares that may be issued pursuant to this delegation of authority will be set by the Board of Directors and must be at least equal to the average weighted by the Company share price volumes on the Euronext Growth Paris market over the last five (5) trading sessions preceding the pricing of the issue, potentially reduced by a maximum discount of 25%. |
62
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 10. Issue of ordinary shares or other securities giving access to the share capital of the Company, without the preferential subscription rights of shareholders, by way of a public offering referred to in Article L.411-2 1° of the French Monetary and Financial Code (fourth resolution of the EGM of December 15, 2022). |
€4,000,000 | February 15, 2025 | None. | The issue price of the shares that may be issued pursuant to this delegation of authority will be set by the Board of Directors and must be at least equal to the average weighted by the Company share price volumes on the Euronext Growth Paris market over the last five (5) trading sessions preceding the pricing of the issue, potentially reduced by a maximum discount of 25%. |
63
| Delegations of authority granted to the the Extraordinary General Meeting of Shareholders |
Maximum nominal amount
of capital increase |
Expiration of the delegation of authority |
Use of delegations of authority granted by the Board of Directors/ Number of shares issued |
Price determination methods | ||||
| 11. Issue of ordinary shares, without preferential subscription rights, to categories of persons meeting specific characteristics (fifth resolution of the EGM of December 15, 2022)(6). |
€4,000,000 | June 15, 2024 | €717,702/717,702 shares February 17, 2023) | The issue price of the shares that may be issued pursuant to this delegation of authority will be set by the Board of Directors and must be at least equal to the average weighted by the Company share price volumes on the Euronext Growth Paris market over the last five (5) trading sessions preceding the pricing of the issue, potentially reduced by a maximum discount of 25%. |
| (1) | The preferential subscription rights of shareholders to the securities covered by this resolution are waived without the designation of specific beneficiaries; |
| (2) | The preferential subscription rights of shareholders to the securities covered by this resolution are waived by way of an offer to qualified investors or to a restricted circle of investors referred to in Article L.411-2-1° of the French Monetary and Financial Code; |
| (3) | The preferential subscription rights of shareholders to the securities covered by this resolution are waived in favor of designated categories of investors; |
| (4) | Free share allocations carried out under this authorization may not give entitlement to a total number of shares exceeding 10% of the number of shares comprising the share capital calculated at the date of allocation; |
| (5) | Share subscription and purchase options granted under this authorization may not give entitlement to a total number of shares exceeding 10% of the number of shares comprising the share capital calculated at the date of allocation; |
| (6) | The preferential subscription rights of shareholders to the securities covered by this resolution are waived in favor of categories of persons meeting specific characteristics. |
64
| 14.3 | AGREEMENTS ENTERED INTO WITH CORPORATE OFFICERS |
| 14.3.1 | Related-party agreements |
The related-party agreements referred to in Articles L.225-38 et seq. of the French Commercial Code are described in chapter 11 of the management report, which contains a list of all agreements authorized by the Board of Directors under the provisions of Articles L.225-38 et seq. of the French Commercial Code.
No other agreements have been concluded between the Company and its corporate officers other than those referred to in this chapter.
| 14.3.2 | Agreements between a corporate officer of the Company or a shareholder holding more than 10% and a company in which Pherecydes Pharma holds, directly or indirectly, more than half of the share capital |
None.
CHAPTER 15—OBSERVATIONS OF THE BOARD OF DIRECTORS
At its meeting of March 30, 2023, the Board of Directors of the Company audited the annual financial statements for the fiscal year ended December 31, 2022.
During this meeting, the Company’s Statutory Auditor presented a summary of their audit on the said statements, which resulted in a report without observations. In addition, the Audit Committee, composed of two members of the Board of Directors, one of whom is independent, presented its conclusions, which corroborate those of the statutory auditor.
In this context and after having deliberated, the Board of Directors unanimously indicated that it had no particular observations to make, either with regard to the Management Board report on the management of the Company or with regard to the annual financial statements for the fiscal year ended December 31, 2022.
Nantes, France,
March 30, 2023
The Board of Directors
65
ANNUAL FINANCIAL STATEMENTS FOR THE FISCAL YEAR ENDED DECEMBER 31, 2022
67
PHERECYDES PHARMA
Table of contents
| Balance sheet – Assets |
1 | |||
| Balance sheet – Liabilities |
2 | |||
| Income statement |
3 | |||
| Income statement (continued) |
4 | |||
| Explanatory notes |
5 |
| CPA AUDIT |
PHERECYDES PHARMA Financial situation as of 12/31/2022
Balance sheet – Assets
| From 01/01/2022 to 12/31/2022 | To 12/31/2021 | |||||||||||||||
| Balance sheet – Assets |
Gross | Depr. Amort. Prov. | Net | Net | ||||||||||||
| Uncalled capital |
||||||||||||||||
| Fixed assets |
||||||||||||||||
| Intangible assets |
||||||||||||||||
| Preliminary and formation expenses |
||||||||||||||||
| Development costs |
5,554,789 | 277,739 | 5,277,049 | |||||||||||||
| Concessions, patents and similar rights |
123,633 | 92,550 | 31,083 | 37,253 | ||||||||||||
| Goodwill |
||||||||||||||||
| Other intangible assets |
5,027,058 | 1,263,418 | 3,763,640 | 6,209,991 | ||||||||||||
| Advances and prepayments on intangible assets |
||||||||||||||||
| Property, plant and equipment |
||||||||||||||||
| Land |
||||||||||||||||
| Buildings |
||||||||||||||||
| Industrial fixtures, fittings, plant machinery & equipment |
721,995 | 586,901 | 135,094 | 143,476 | ||||||||||||
| Other tangible fixed assets |
839,799 | 415,709 | 424,090 | 519,551 | ||||||||||||
| Assets under construction |
||||||||||||||||
| Advances and prepayments |
113,538 | 113,538 | ||||||||||||||
| Financial fixed assets |
||||||||||||||||
| Shares in associated companies |
||||||||||||||||
| Other equity investments |
||||||||||||||||
| Loans to group companies |
||||||||||||||||
| Long-term investments |
||||||||||||||||
| Other long-term securities |
50,930 | 25,661 | 25,269 | 105,673 | ||||||||||||
| Loans |
||||||||||||||||
| Other financial fixed assets |
124,556 | 124,556 | 137,040 | |||||||||||||
| FIXED ASSETS |
12,556,297 | 2,775,515 | 9,780,782 | 7,152,984 | ||||||||||||
| Current assets |
||||||||||||||||
| Inventories of products and work in progress |
||||||||||||||||
| Raw materials, supplies and other consumables |
||||||||||||||||
| Work in progress: |
||||||||||||||||
| – For production |
||||||||||||||||
| – For services |
||||||||||||||||
| Intermediate and finished goods |
||||||||||||||||
| Inventories of goods purchased for resale |
||||||||||||||||
| Advances and prepayments paid on orders |
||||||||||||||||
| Receivables |
||||||||||||||||
| Trade accounts receivable |
126,604 | 126,604 | ||||||||||||||
| Other |
2,241,186 | 2,241,186 | 1,552,905 | |||||||||||||
| Capital called up and unpaid |
||||||||||||||||
| Marketable securities |
||||||||||||||||
| Company own shares |
||||||||||||||||
| Other securities |
||||||||||||||||
| Liquid debts |
||||||||||||||||
| Cash and cash equivalents |
1,035,127 | 1,035,127 | 5,357,080 | |||||||||||||
| Prepaid expenses |
32,412 | 32,412 | 21,904 | |||||||||||||
| CURRENT ASSETS |
3,435,329 | 3,435,329 | 6,931,889 | |||||||||||||
| Debt issuance costs to be spread over several periods |
||||||||||||||||
| Premiums arising from bond redemptions |
||||||||||||||||
| Unrealized exchange loss |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL ASSETS |
15,991,626 | 2,775,515 | 13,216,110 | 14,084,872 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| CPA AUDIT | Page 1 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
Balance sheet – Liabilities
| From 01/01/2022 | From 01/01/2021 | |||||||
| Balance sheet – Liabilities |
To 12/31/2022 | To 12/31/2021 | ||||||
| Shareholders’ equity |
||||||||
| Equity capital (of which paid up) 7,221,477 |
7,221,477 | 5,852,308 | ||||||
| Premiums arising from share issues, mergers, assets brought into business |
8,903,830 | 7,117,723 | ||||||
| Revaluation reserves |
||||||||
| Equity method evaluation difference |
||||||||
| - Legal |
||||||||
| - Statutory or contractual Reserves: |
||||||||
| - Regulated |
||||||||
| - Other |
9,475,980 | 9,483,148 | ||||||
| Profits/losses brought forward |
-13224788 | -10035731 | ||||||
| Result for the year (profit or loss) |
-4,854,342 | -3189057 | ||||||
| Investment grants |
||||||||
| Regulated provisions |
||||||||
| SHAREHOLDERS’ EQUITY |
7,522,158 | 9,228,391 | ||||||
| Other liable equity capital |
||||||||
| Proceeds from issues of equity securities |
||||||||
| Conditional advances |
531,882 | 766,667 | ||||||
| Other |
||||||||
| OTHER LIABLE EQUITY CAPITAL |
531,882 | 766,667 | ||||||
| Provisions for contingencies and charges |
||||||||
| Provisions for: |
||||||||
| - Contingencies |
||||||||
| - Charges |
||||||||
| PROVISIONS FOR CONTINGENCIES AND CHARGES |
||||||||
| Loans and debt |
||||||||
| Convertible debenture loans |
9,484 | 23,558 | ||||||
| Other debenture loans |
||||||||
| - Loans and debt: From credit institutions: |
360,000 | 400,000 | ||||||
| - From various lenders |
2,168,780 | 2,000,000 | ||||||
| Advances and prepayments received for orders in progress |
||||||||
| Financial borrowings and debt: |
||||||||
| - Trade accounts payable |
1,589,993 | 784,409 | ||||||
| - Tax and payroll |
791,278 | 881,847 | ||||||
| - Fixed assets |
||||||||
| Other debts |
2,535 | |||||||
| Liquid debts |
||||||||
| Deferred income |
240,000 | |||||||
| LOANS AND DEBT |
5,162,071 | 4,089,814 | ||||||
| Unrealized exchange profit |
||||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES |
13,216,110 | 14,084,872 | ||||||
|
|
|
|
|
|||||
| CPA AUDIT | Page 2 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
Income statement
| From 01/01/2022 to 12/31/2022 |
|
|
As of 12/31/2021 | |||||||||||||
| Income statement |
France | Exportation | Total | Total | ||||||||||||
| Operating income |
||||||||||||||||
| Sales of goods |
||||||||||||||||
| Production sold: |
||||||||||||||||
| - For production |
||||||||||||||||
| - For services |
||||||||||||||||
| Net revenue |
||||||||||||||||
| Production: |
||||||||||||||||
| - Inventoried |
||||||||||||||||
| - Capitalized |
3,155,303 | 2,142,291 | ||||||||||||||
| Operating grants received |
||||||||||||||||
| Reversals of amortization, depreciation, impairment and provisions, transfers of expenses |
5,140 | 58,025 | ||||||||||||||
| Other income |
6,792 | 65 | ||||||||||||||
| OPERATING INCOME |
3,167,235 | 2,200,380 | ||||||||||||||
| Operating expenses |
||||||||||||||||
| Purchases of goods (including customs duties) |
||||||||||||||||
| Change in inventory (goods) |
||||||||||||||||
| Purchases of raw materials, supplies and other consumables |
||||||||||||||||
| Change in inventory (raw materials, supplies and other consumables) |
||||||||||||||||
| Other external expenses and purchases* |
5,960,963 | 3,386,273 | ||||||||||||||
| Taxes and similar levies |
79,361 | 27,678 | ||||||||||||||
| Wages and salaries |
2,330,672 | 2,015,555 | ||||||||||||||
| Social security contributions |
990,671 | 893,130 | ||||||||||||||
| Charges for: |
||||||||||||||||
| - Depreciation or amortization on fixed assets |
444,423 | 158,731 | ||||||||||||||
| - Impairment of fixed assets |
||||||||||||||||
| - Impairment of current assets |
||||||||||||||||
| - Provisions for contingencies and charges |
||||||||||||||||
| Other charges |
51,188 | 39,708 | ||||||||||||||
| OPERATING EXPENSES |
9,857,278 | 6,521,075 | ||||||||||||||
| * Including: |
||||||||||||||||
| - Equipment leasing royalties |
100,566 | 58,217 | ||||||||||||||
| - Property leasing royalties |
||||||||||||||||
| OPERATING RESULTS |
-6,690,043 | -4,320,694 | ||||||||||||||
| Profit allocated or loss transferred |
||||||||||||||||
| Loss incurred or profit transferred |
||||||||||||||||
| Financial income |
||||||||||||||||
| Financial income from investments |
||||||||||||||||
| Income from other securities and fixed asset receivables |
||||||||||||||||
| Other interest and similar income |
921 | 39 | ||||||||||||||
| Reversals of provisions, impairment, and transfers of expenses |
||||||||||||||||
| Positive exchange rate differences |
86 | |||||||||||||||
| Net income from disposals of marketable securities |
||||||||||||||||
| FINANCIAL INCOME |
1,007 | 39 | ||||||||||||||
| Financial expenses |
||||||||||||||||
| Financial allowances for amortization, depreciation, impairment and provisions |
11,833 | 13,828 | ||||||||||||||
| Interest and similar expenses |
52,661 | 32,558 | ||||||||||||||
| Negative exchange rate differences |
17 | |||||||||||||||
| Net expenses from disposals of marketable securities |
||||||||||||||||
| FINANCIAL EXPENSES |
64,511 | 46,386 | ||||||||||||||
| FINANCIAL RESULTS |
-63,504 | -46,346 | ||||||||||||||
| CURRENT RESULTS BEFORE TAXES |
-6,753,546 | -4,367,040 | ||||||||||||||
| CPA AUDIT | Page 3 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
Income statement (continued)
| Income statement (continued) |
As of 12/31/2022 | As of 12/31/2021 | ||||||
| Extraordinary income |
||||||||
| On management operations |
240,000 | |||||||
| On capital transactions |
355,974 | 345,700 | ||||||
| Reversals of provisions, impairment, and transfers of expenses |
||||||||
| EXTRAORDINARY INCOME |
595,974 | 345,700 | ||||||
| Extraordinary expenses |
||||||||
| On management operations |
15,000 | |||||||
| On capital transactions |
84,849 | 27,692 | ||||||
| Extraordinary allowances for amortization, depreciation, impairment and provisions |
113,538 | |||||||
| EXTRAORDINARY EXPENSES |
84,849 | 156,230 | ||||||
| EXTRAORDINARY RESULTS |
511,125 | 189,470 | ||||||
| Employee shareholding |
||||||||
| Income tax |
-1,388,080 | -988,514 | ||||||
|
|
|
|
|
|||||
| TOTAL INCOME |
3,764,216 | 2,546,119 | ||||||
|
|
|
|
|
|||||
| TOTAL EXPENSES |
8,618,558 | 5,735,176 | ||||||
|
|
|
|
|
|||||
| PROFIT OR LOSS (Total income - Total expenses) |
-4,854,342 | -3,189,057 | ||||||
|
|
|
|
|
|||||
| CPA AUDIT | Page 4 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
Explanatory notes
The balance sheet before distribution for the period ended December 31, 2022, which totals €13,216,110, and the income statement for the fiscal year, which shows a profit of –€4,854,342, presented in the form of a list.
This current financial situation covers a period of 12 months, from 01/01/2022 to 12/31/2022. To facilitate comparative reading:
| • | The balance sheet as of December 31, 2022 has been compared to December 31, 2021. |
| • | The income statement as of December 31, 2022 has been compared to December 31, 2021. |
The following notes and tables are an integral part of the financial situation.
| CPA AUDIT | Page 5 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
TABLE OF CONTENTS
TYPE OF ACTIVITY
BASIS FOR THE PREPARATION OF THE ANNUAL FINANCIAL STATEMENTS AND ACCOUNTING PRINCIPLES
| • | Accounting principles and general conventions |
| • | Going concern principles |
| • | Judgments and estimates of the Management of the Company |
| • | Financial statements reporting currency |
SIGNIFICANT EVENTS AND INFORMATION NECESSARY FOR A FAIR PRESENTATION OF RESULTS
| • | Significant events during the reporting period |
| • | Significant events after the reporting period |
ACCOUNTING METHODS AND PRINCIPLES
NOTES TO THE ANNUAL FINANCIAL STATEMENTS
| • | Statement of fixed assets |
| • | Statement of amortization and depreciation |
| • | Analysis of maturities and receivables |
| • | Accrued income and receivables |
| • | Share capital and changes in equity |
| • | Potential share capital |
| • | Conditional advances |
| • | Statement of provisions |
| • | Analysis and maturities of liabilities |
| • | Accrued expenses and credit notes to be issued |
| • | Deferred income and expenses |
| • | Capitalized production |
| • | Other external expenses and purchases |
| • | Non-recurring income from operations |
| • | Corporate tax and loss carryforwards |
OTHER ADDITIONAL INFORMATION
| • | Average workforce |
| • | Off-balance sheet commitments |
| • | Related parties |
| CPA AUDIT | Page 6 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 1. | Type of activity |
Pherecydes Pharma conducts research and development activity in the medical field. This includes:
| • | developing expertise; |
| • | obtaining patents and licenses in the field of biology, medicine and, more generally, in the field of life sciences on its own behalf or on behalf of third parties with a view to the marketing and distribution of its products; |
| • | any service activity related to the life sciences field. |
| 2. | Basis for the preparation of the annual financial statements and accounting principles |
ACCOUNTING PRINCIPLES AND GENERAL CONVENTIONS
The annual financial statements have been prepared in accordance with generally accepted accounting principles in France and in compliance with the provisions of ANC regulation number 2014-03, the French Commercial Code and French Decree no. 83-1020 of November 29, 1983.
These financial statements were approved by the board of directors on March 30, 2023.
The general accounting conventions have been applied with regard to the principle of prudence, in accordance with the basic assumptions:
| • | going concern (as defined in Article L.123-20 of the French Commercial Code); |
| • | consistency of accounting methods from one fiscal year to the next; |
| • | intangibility of the opening balance sheet, |
| • | independence of fiscal years; |
and in accordance with the general rules governing the preparation and presentation of annual financial statements.
The basic method used to value items entered in the accounts is the historical cost method.
GOING CONCERN PRINCIPLE
The company’s cash balance on December 31, 2022 amounts to €1,035,127.
The going concern principle of accounting has been adopted in view of the following elements:
| • | The Company’s historical deficit position is explained by the innovative nature of the products under development, which involves a research and development phase lasting several years. |
| • | The outlook for the Company’s growth over the next twelve months, on which the budget is based, are considered reasonable in the light of the Company’s recent development. |
| • | On September 22, 2022, Pherecydes Pharma successfully carried out a capital increase of €3.1 million, of which €2.6 million from institutional and historical investors and €0.5 million from individuals, via the PrimaryBid platform. |
| • | On February 15, 2023, Pherecydes Pharma and ERYTECH Pharma (“ERYTECH”; Nasdaq & Euronext: ERYP), a clinical-stage biopharmaceutical company developing innovative therapies by encapsulating therapeutic drug substances inside red blood cells, announced their proposed strategic combination intended to create a global player in extended phagotherapy and accelerate the development of a portfolio of drug candidates targeting pathogenic bacteria and potential other indications of high unmet medical needs. |
The strategic combination would build on the complementary expertise and capabilities of both companies to accelerate the development of extended phagotherapies for antimicrobial resistance, in particular via the PhagoDAIR phase II study conducted by PHERECYDES, as well as other anti-infective fields and therapeutic areas with high unmet medical needs.
The cash runway of the new entity resulting from the merger would extend into the third quarter of 2024, with a consolidated cash position of approximately €41 million as of December 31, 2022, and would enable funding of existing and novel programs through multiple clinical milestones.
This transaction would pave the way for the expansion of PHERECYDES development programs in antimicrobial resistance and complete the strategic review process announced by ERYTECH on several occasions since November 2021.
The transaction expected to close at the end of the second quarter of 2023.
The transaction, supported by the key shareholders of PHERECYDES and ERYTECH, will result in former PHERECYDES shareholders holding approximately 49% of the combined entity.
ERYTECH and PHERECYDES intend to merge their operations and relocate all teams to ERYTECH’s premises in Lyon, France, where they will benefit from being located within a major European center for infectious diseases.
| • | On February 17, 2023, Pherecydes Pharma successfully completed a €1.5 million capital increase |
| • | At the same time, and to meet its cash needs to finance its activities for the next twelve months, the Company is seeking additional financing solutions of various kinds, including equity financing and simple or bonded debt, from third parties or its shareholders, as well as grants and/or repayable advances specifically related to the Company’s research programs. |
| CPA AUDIT | Page 7 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
JUDGMENTS AND ESTIMATES OF THE MANAGEMENT OF THE COMPANY
The preparation of financial statements requires management to make judgments, estimates and assumptions that have an impact on the amounts of assets and liabilities, as well as on the income statement items for the period. These estimates consider economic data that may change over time and include contingencies.
The estimates and underlying assumptions are based on past experience and other factors considered reasonable in light of current and anticipated circumstances and the economic environment. They thus serve as a basis for exercising the judgment required to determine the book value of assets and liabilities that cannot be obtained directly from other sources. Actual values may differ from estimated values.
The estimates and underlying assumptions are reviewed on an ongoing basis. The principal estimates are related to:
| • | The life of fixed assets; |
| • | The value of capitalized development costs; |
| • | The value of capitalized production; |
| • | The Research Tax Credit. |
FINANCIAL STATEMENTS REPORTING CURRENCY
The financial statements, as well as all the notes and tables presented in these explanatory notes, are expressed in euros.
| 3. | Significant events and information necessary for a fair presentation of results |
| 3.1. | Significant events during the reporting period from January 1 to December 31, 2022 |
| • | Obtaining a €2.1 million grant for the PhagECOLI project conducted in partnership with the CEA |
In January 2022, the company announced that the PhagECOLI project was selected for the “Emerging infectious diseases and new RBC threats”
Call for Expressions of Interest. Within this framework, the partnership will receive grants and repayable advances for an amount of €2.1 million over the three years of the project’s implementation.
| • | Obtaining CPP approval to initiate the PhagoDAIR program |
In January 2022, the company received CPP approval for the PhagoDAIR study. With this green light, the Company has all the regulatory approvals to initiate this phase I/II study.
| • | Capital increases |
The company carried out four capital increases during the first half of 2022:
| • | By allocating 7,168 free shares on February 4, 2022 with a nominal value of €1, i.e. a capital increase of €7,168. |
| • | By exercising a start-up stock option (BSPCE) in April 2022 for a total of 10,116 shares with a nominal value of €1, which resulted in a capital increase of €10,116 and a total share issue premium of €10,470.06 |
| • | By exercising a start-up stock option in July 2022 for a total of 5,396 shares with a nominal value of €1, which resulted in a capital increase of €5,396 and a total share issue premium of €5,584.86 |
| • | By exercising a start-up stock option in August 2022 for a total of 25,659 shares with a nominal value of €1, which resulted in a capital increase of €25,659 and a total share issue premium of €26,557.06 |
| • | By fundraising in September 2022 for a total of 1,320,830 shares with a nominal value of €1, which resulted in a capital increase of €1,320,830 and a total share issue premium of €1,743,495. |
| CPA AUDIT | Page 8 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| • | Conclusion of a grant and repayable advance contract |
In February 2022, BPIFRANCE entered into a grant and repayable advance agreement with Pherecydes Pharma to finance the PhagECOLI program for an amount of €1,687,800. This financing will be 60% in the form of a grant and 40% in the form of a repayable advance, to be paid after each milestone and conditional on Pherecydes Pharma providing a certain number of elements.
The advance is repayable quarterly starting on December 31, 2026, unless the program is unsuccessful.
As of December 31, 2022, €169 thousand have been received in repayable advances, and €253 thousand in grants.
| 3.2. | Significant events after the reporting period |
| • | Capital increase |
On February 22, 2023, management decided to carry out a capital increase through the issue of 717,702 new shares with a nominal value of €1 each and a total share issue premium of €782,295.18 (i.e., a share issue premium of €1.09 per ordinary share.)
| • | Strategic combination with Erytech |
On February 15, 2023, Pherecydes Pharma and ERYTECH Pharma (“ERYTECH”; Nasdaq & Euronext: ERYP), a clinical-stage biopharmaceutical company developing innovative therapies by encapsulating therapeutic drug substances inside red blood cells, announced their proposed strategic combination intended to create a global player in extended phagotherapy and accelerate the development of a portfolio of drug candidates targeting pathogenic bacteria and potential other indications of high unmet medical needs.
| 4. | Accounting methods and principles |
| 4.1. | RESEARCH AND DEVELOPMENT COSTS |
The capitalized development costs relate to clearly individualized subjects with a high probability of technical success and commercial profitability or economic viability. Pherecydes Pharma has considered as eligible for capitalization the development in-progress costs of products for which the regulatory authorities have validated the Company’s good manufacturing practices or have given the Company authorization to proceed with human clinical trials and/or Early Access Approval (AAC).
As of December 31, 2022, the Company has capitalized research and development expenses for a gross value of €5,554,789. See notes 5.12 and 5.14.
| CPA AUDIT | Page 9 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 4.2. | PROPERTY, PLANT AND EQUIPMENT AND INTANGIBLE ASSETS |
Intangible assets
Intangible assets are recorded at their initial acquisition cost minus accumulated amortization and impairment losses.
All intangible assets have a finite life and are amortized over their estimated useful lives using the straight-line method on the following bases:
| • | Patents: 20 years |
However, an impairment test is performed whenever there is an indication of impairment loss.
In the income statement, depreciation, amortization, and impairment charges on intangible assets are recorded under “Depreciation or amortization charges on fixed assets” or “Provisions on fixed assets.”
Property, plant, and equipment
Property, plant, and equipment are valued at acquisition or production cost, taking into account the costs required to bring the assets into use and after deducting trade discounts, rebates and cash discounts obtained, minus accumulated depreciation.
All property, plant and equipment are depreciated over their useful lives using the straight-line method on the following bases:
| Industrial machinery | 6 years | |
| Work and installations | from 3 to 10 years | |
| Office and IT equipment | from 2 to 5 years | |
| Furniture | 5 years |
In the income statement, depreciation charges on property, plant and equipment are recorded under “Depreciation or amortization charges on fixed assets.”
| 4.3. | FINANCIAL ASSETS |
The gross value is the purchase cost excluding incidental expenses. When the inventory value is lower than the gross value, an impairment is recorded for the difference.
| 4.4. | RECEIVABLES AND FINANCIAL BORROWINGS AND DEBT |
Receivables and payables are valued at their nominal value. An impairment is recorded when the inventory value is lower than the book value.
| 4.5. | CASH AND CASH EQUIVALENTS |
Cash and cash equivalents include those in banks and in hand. Cash and cash equivalents are valued at their nominal value.
| 4.6. | OTHER LIABLE EQUITY CAPITAL |
The Company benefits from three conditional advances granted by BPI France. Repayment of this advance is conditional on the success of the subsidized project.
The balance of the conditional advances is reduced by the repayments made according to the contractual schedule.
For easier reading, these advances, which appeared on the “Other” line of the “Other liable equity capital” item, have been repositioned on the “Conditional advances” line of this same item in the company’s balance sheet on December 31, 2022.
| 4.7. | OTHER LOANS AND FINANCIAL DEBT |
For easier reading, the loans taken out with BPI, which appeared on the “Loans and debt from credit institutions” line have been repositioned on the “Other loans and financial debt” line in the company’s balance sheet on December 31, 2022.
| CPA AUDIT | Page 10 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 4.8. | GRANTS |
The grants received are investment grants insofar as they finance long-term activities.
When these grants are definitively acquired by the company (i.e., there is no risk of them being challenged or needing to be repaid, in particular through conditions precedent), they are recognized, in proportion to the progress of the subsidized study:
| • | either as extraordinary income, if the financed expenses have been recorded as expenses (recognized for the share of the expenses incurred); |
| • | or as shareholders’ equity if the financed expenses have been capitalized (recognized over the same period and at the same rate as the fixed asset). |
Where applicable, they are recognized as advances and prepayments received under liabilities for financial borrowings and debt.
| 4.9. | EXCEPTIONAL RESULTS |
Income and charges which, by their nature or occurrence, are not part of the Company’s regular activities, are recognized as extraordinary results.
| 4.10. | PENSION LIABILITIES |
The only pension liability borne by the Company is that of retirement bonuses for employees. Legislation requires a bonus to be paid to employees when they retire based on their length of service and their salary at retirement age.
The Company has decided not to make any provision in its financial statements for retirement bonuses or pension supplements for its employees. The corresponding expense is recorded in the period during which the bonus is actually paid. The liabilities are presented as off-balance sheet commitments. See note 5.16.
| 5. | NOTES TO THE ANNUAL FINANCIAL STATEMENTS |
| 5.1. | Statement of fixed assets |
| Framework A |
Gross value at beginning of fiscal year |
Increases | ||||||||||
| Intangible assets |
Revaluation for the fiscal year |
Acquisitions, receivables, transfers |
||||||||||
| Research and development costs |
5,554,789 | |||||||||||
| Other intangible asset items concessions patents |
7,597,042 | 3,108,438 | ||||||||||
|
|
|
|
|
|||||||||
| TOTAL |
7,597,042 | 8,663,227 | ||||||||||
|
|
|
|
|
|||||||||
| Property, plant, and equipment |
||||||||||||
| Land |
||||||||||||
| Buildings on own land Buildings on land owned by third parties Building general facilities, fixtures, and fittings |
||||||||||||
| Industrial fixtures, fittings, plant machinery & equipment |
672,286 | 49,709 | ||||||||||
| Other general facilities, fixtures, and fittings Transportation equipment Office and IT equipment, furniture |
596,504 | |||||||||||
| Reusable packaging materials and other |
236,333 | 6,963 | ||||||||||
| Tangible assets in progress |
||||||||||||
| Advances and prepayments |
113,538 | |||||||||||
|
|
|
|||||||||||
| TOTAL |
1,618,660 | 56,671 | ||||||||||
|
|
|
|
|
|||||||||
| Financial fixed assets |
||||||||||||
| Shares in associated companies Other equity investments Other long-term securities |
119,501 | |||||||||||
| Loans and other financial fixed investments |
137,040 | 3,421 | ||||||||||
|
|
|
|
|
|||||||||
| TOTAL |
256,541 | 3,421 | ||||||||||
|
|
|
|
|
|||||||||
| OVERALL TOTAL |
9,472,244 | 8,723,319 | ||||||||||
|
|
|
|
|
|||||||||
| CPA AUDIT | Page 11 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| Framework B |
Decreases | Gross value of fixed assets at end of fiscal year |
Statutory reval. or valu. using the equity method |
|||||||||||||
| Transfer | Disposal | Original value of fixed assets at end of fiscal year |
||||||||||||||
| Research and development costs |
5,554,789 | |||||||||||||||
| Other intangible asset items |
5,554,789 | 5,150,690 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| TOTAL |
5,554,789 | 10,705,479 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Land |
||||||||||||||||
| Buildings on own land |
||||||||||||||||
| Buildings on land owned by third parties Building general facilities, fixtures, and fittings Industrial fixtures, fittings, plant machinery & equipment |
721,995 | |||||||||||||||
| Other general facilities, fixtures, and fittings |
596,504 | |||||||||||||||
| Transportation equipment Office and IT equipment, furniture |
243,295 | |||||||||||||||
| Reusable packaging materials and other |
||||||||||||||||
| Tangible assets in progress |
||||||||||||||||
| Advances and prepayments |
113,538 | |||||||||||||||
|
|
|
|||||||||||||||
| TOTAL |
1,675,332 | |||||||||||||||
|
|
|
|||||||||||||||
| Shares in associated companies |
||||||||||||||||
| Other equity investments |
||||||||||||||||
| Other long-term securities |
68,571 | 50,930 | ||||||||||||||
| Loans and other financial fixed investments |
15,904 | 124,556 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| TOTAL |
84,475 | 175,486 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| OVERALL TOTAL |
5,554,789 | 84,475 | 12,556,297 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| CPA AUDIT | Page 12 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
The gross value of research and development costs amounts to €10,705 thousand, of which €5,151 thousand in assets under construction and €5,555 thousand in intangible assets made available during the anti-Staphylococcus aureus program.
As of December 31, 2022, the gross costs recorded as assets under construction amounted to €5,151 thousand. They mainly correspond to development costs for various assets:
| • | PSEUDO for total gross amount of €3,778 thousand |
| • | PHAGOBURN for total gross amount of €1,263 thousand |
Other intangible assets correspond to patents. The Company has four patent families (two for anti-Pseudomonas aeruginosa phages, one for anti-Staphylococcus aureus phages and one for anti-E. coli phages). These patents have been granted in some jurisdictions and are pending in others.
| CPA AUDIT | Page 13 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 5.2. | Statement of amortization and depreciation and impairment |
| Framework A | ||||||||||||||||
| SITUATIONS AND MOVEMENTS DURING THE FISCAL YEAR |
||||||||||||||||
| DEPRECIABLE OR AMORTIZABLE ASSETS |
Amount at the beginning of the fiscal year |
Increase | Decrease | Amount at the end of the fiscal year |
||||||||||||
| Intangible assets | ||||||||||||||||
| Research and development costs |
277,739 | 277,739 | ||||||||||||||
| Software |
86,380 | 6,170 | 92,550 | |||||||||||||
| R&D cost provision |
1,263,418 | 1,263,418 | ||||||||||||||
| Assets under construction |
113,537 | 113,537.6 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL |
1,463,335 | 283,909 | 1,747,245 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Property, plant, and equipment |
||||||||||||||||
| Land |
||||||||||||||||
| Buildings on own land |
||||||||||||||||
| Buildings on land owned by third parties |
||||||||||||||||
| Building general facilities, fixtures, and fittings |
||||||||||||||||
| Industrial fixtures, fittings, plant machinery & equip. |
528,810 | 58,090 | 586,901 | |||||||||||||
| Other general facilities, fixtures, and fittings |
182,733 | 58,414 | 241,147 | |||||||||||||
| Transportation equipment |
||||||||||||||||
| Office and IT equipment, furniture |
130,553 | 44,009 | 174,562 | |||||||||||||
| Reusable packaging materials and other |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL |
842,096 | 160,514 | 1,002,609 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| OVERALL TOTAL |
2,305,431 | 444,423 | 2,749,854 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The impairment of €1,263 thousand corresponds to expenses related to the Phagoburn project, which stopped in 2018. As the analyses performed in 2018 showed negative results, the phages were not usable and therefore the project could not, in its current state, generate future revenue. As a result, the total amount of capitalized costs on this project had been impaired in 2018, as a matter of prudence, pending the possible resumption of the project.
| CPA AUDIT | Page 14 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 5.3. | Analysis of maturities and receivables |
| Receivables(a) |
Gross amount | Asset liquidity | ||||||||||
| Maturities of less than 1 year |
Maturities of more than 1 year |
|||||||||||
| Non-current assets |
||||||||||||
| Loans to group companies |
||||||||||||
| Loans (1)(2) |
||||||||||||
| Other financial fixed assets |
124,556 | 124,556 | ||||||||||
| Current assets |
||||||||||||
| Doubtful or disputed receivables |
||||||||||||
| Other trade accounts receivable |
126,604 | 126,604 | ||||||||||
| Receivables on loaned securities |
||||||||||||
| Personnel and related expenses |
2,171 | 2,171 | ||||||||||
| Social security and related liabilities |
3,717 | 3,717 | ||||||||||
| Income tax |
1,388,080 | 1,388,080 | ||||||||||
| Value added tax |
345,305 | 345,305 | ||||||||||
| Other taxes and similar levies |
||||||||||||
| Other |
||||||||||||
| Trade accounts payable to group companies and associates (2) |
||||||||||||
| Other receivables (including receivables from repurchase agreements) |
479,698 | 479,698 | ||||||||||
| Prepaid expenses |
32,412 | 32,412 | ||||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL |
2,502,543 | 2,377,978 | 124,556 | |||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Loans granted during the year |
(1) Repayments received during the year
(2) Loans and advances granted to individuals
| 5.4. | Accrued income and receivables |
(Article R.123-189 of the French Commercial Code)
| ACCRUED INCOME INCLUDED IN THE |
Fiscal year ended | Fiscal year ended | ||||||
| 12/31/2022 | 12/31/2021 | |||||||
| Loans to group companies |
||||||||
| Other long-term securities |
||||||||
| Loans |
||||||||
| Other financial fixed assets |
||||||||
| Trade accounts receivable |
||||||||
| Other accounts receivable |
3,717 | 3,063 | ||||||
| Marketable securities |
||||||||
| Cash and cash equivalents |
||||||||
|
|
|
|
|
|||||
| TOTAL |
3,717 | 3,063 | ||||||
|
|
|
|
|
|||||
| CPA AUDIT | Page 15 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 5.5. | Share capital and changes in equity |
On December 31, 2022, the share capital amounted to €7,221,477.
It is divided into 7,221,477 shares with a nominal value of €1 each
The change in share capital over the period is as follows:
| Quantity | Value | |||||||||||||||||||||||
| Shareholders |
Ordinary shares |
P1 shares | Q2 Share subscription warrants (BSA T2) |
Total shares | Security value | Share capital amount |
||||||||||||||||||
| 12/31/2021 |
5,852,308 | — | — | 5,852,308 | € | 1.00 | € | 5,852,308 | ||||||||||||||||
| 12/31/2022 |
7,221,477 | — | — | 7,221,477 | € | 1.00 | € | 7,221,477 | ||||||||||||||||
| Change |
1,369,169 | — | — | 1,369,169 | € | 1.00 | € | 1,369,169 | ||||||||||||||||
The table of changes in shareholders’ equity is as follows:
| in euros |
12/31/2021 | Allocation of income |
Exercise of start-up stock options |
Free share allocation |
Capital increase (fundraising) |
12/31/2022 | ||||||||||||||||||
| Share capital |
5,852,308 | 41,171 | 7,168 | 1,320,830 | 7,221,477 | |||||||||||||||||||
| Premiums arising from share issues, mergers, assets brought into business |
7,117,723 | 42,612 | 1,743,495 | 8,903,830 | ||||||||||||||||||||
| Statutory reserves |
— | |||||||||||||||||||||||
| Other reserves |
9,483,148 | -7,168 | 9,475,980 | |||||||||||||||||||||
| Profits/losses brought forward |
-10,035,731 | -3,189,057 | -13,224,788 | |||||||||||||||||||||
| Result for the fiscal year |
-3,189,057 | 3,189,057 | -4,854,342 | |||||||||||||||||||||
| Shareholders’ equity |
9,228,391 | — | 83,783 | — | 3,064,325 | 7,522,158 | ||||||||||||||||||
| 5.6. | Potential share capital |
| CPA AUDIT | Page 16 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
The table below details the total number of shares that may be issued by type of financial instrument on December 31, 2022:
| Potential share capital |
Number | Parity | Maximum number of shares to be issued |
|||||||||
| 2017 start-up stock option |
— | 1 for 1 | — | |||||||||
| 2018 start-up stock option |
4,200 | 1 for 1 | 4,200 | |||||||||
| BSPCE 2019 |
29,538 | 1 for 1 | 29,538 | |||||||||
| 2020 start-up stock option |
144,200 | 1 for 1 | 144,200 | |||||||||
| 2021 start-up stock option |
437,977 | 1 for 1 | 437,977 | |||||||||
| 2022 start-up stock option |
— | 1 for 1 | — | |||||||||
| Number of potential shares |
615,915 | |||||||||||
| Number of existing shares |
7,221,477 | |||||||||||
| Overall dilution |
7.85867 | % | ||||||||||
The impact of the overall dilution on the situation of the associates following the exercise of all the instruments detailed above would be:
| Effect of dilution on earnings per share | Situation of the shareholder before |
Situation of the shareholder after |
||||||
| For €1 of earnings per share |
1.000000 | 0.92145736 | ||||||
The characteristics of the capital instruments issued are summarized below.
| • | Start-up stock options: |
The number of start-up stock options on December 31, 2022 is 615,450, or:
| • | 0 x 2017 start-up stock options |
| • | 4,200 x 2018 start-up stock options |
| • | 29,538 x 2019 start-up stock options |
| • | 144,200 x 2020 start-up stock options |
| • | 437,977 x 2021 start-up stock options |
| • | 0 x 2022 start-up stock options |
| 5.7. | Conditional advances |
On December 31, 2022, conditional advances amounted to €531,882, with the following characteristics:
| • | PHAGOGRAM advance received from BPI France |
| • | Amount received: €117,882 |
| • | Balance of the remaining advance receivable: €0 |
| • | PHAGOSCLIN advance received from BPI France |
| • | Amount received: €700,000 |
| • | Amount to be repaid: €414,000 |
| • | The Company was granted a €240,000 debt write-off in the first half of 2022. |
| • | PHAGECOLI advance received from BPI France |
| • | Amount received: €168,780 |
| • | Balance of the remaining advance receivable: €506,340 – pending completion of milestones 1 and 2 |
Estimated repayment schedule:
| Project |
< 1 year | 1 to 5 years | 5 years | |||||||||
| Phagogram |
117,882 | |||||||||||
| Phagosclin |
69,000 | 345,000 | ||||||||||
| PhagECOLI |
168,780 | |||||||||||
|
|
|
|
|
|
|
|||||||
| Total |
69,000 | 631,662 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| CPA AUDIT | Page 17 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 5.8. | Statement of provisions and impairments |
| BREAKDOWN OF PROVISIONS |
Amount at the beginning of the fiscal year |
Increase in charges for the fiscal year |
Decreases reversed at the end of the fiscal year |
Amount at the end of the fiscal year |
||||||||||||
| Regulated |
||||||||||||||||
| Provision for the reconstitution of mining deposits |
||||||||||||||||
| Provisions for capital expenditure |
||||||||||||||||
| Provisions for price increases |
||||||||||||||||
| Accelerated depreciation or amortization |
||||||||||||||||
| Tax Prov. for foreign operations before 01/01/1992 |
||||||||||||||||
| Tax Prov. for foreign operations after 01/01/1992 |
||||||||||||||||
| Provisions for start-up loans |
||||||||||||||||
| Other regulated provisions |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Contingencies and charges |
||||||||||||||||
| Provisions for disputes |
||||||||||||||||
| Provisions for guarantees given to customers |
||||||||||||||||
| Provisions for losses on futures contracts |
||||||||||||||||
| Provisions for fines and penalties |
||||||||||||||||
| Provisions for foreign exchange losses |
||||||||||||||||
| Provisions for pensions and benefit obligations |
||||||||||||||||
| Provisions for taxes |
||||||||||||||||
| Provisions for replacement of fixed assets |
||||||||||||||||
| Provisions for major repairs and maintenance |
||||||||||||||||
| Provisions for social security charges on paid leave |
||||||||||||||||
| Other provisions for contingencies and charges |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| CPA AUDIT | Page 18 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| BREAKDOWN OF IMPAIRMENT LOSSES |
Amount at the beginning of the fiscal year |
Increase in charges for the fiscal year |
Decreases reversed at the end of the fiscal year |
Amount at the end of the fiscal year |
||||||||||||
| Impairment |
||||||||||||||||
| Intangible assets |
1,376,956 | 1,376,956 | ||||||||||||||
| Property, plant, and equipment |
||||||||||||||||
| Fixed assets – investments in associates |
||||||||||||||||
| Fixed assets – investments in subsidiaries and affiliates |
||||||||||||||||
| Financial fixed assets |
13,828 | 61,280 | 49,447 | 25,661 | ||||||||||||
| On inventories and work in progress |
||||||||||||||||
| On trade accounts receivables |
||||||||||||||||
| Other provisions for impairment losses |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| TOTAL |
1,390,783 | 61,280 | 49,447 | 1,402,616 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| OVERALL TOTAL |
1,390,783 | 61,280 | 49,447 | 1,402,616 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Of which charges and reversals: – operational |
||||||||||||||||
| Of which charges and reversals: – financial |
11,833 | |||||||||||||||
| Of which charges and reversals: – extraordinary |
||||||||||||||||
| Investments in associates: Total impairment loss |
|
|||||||||||||||
| CPA AUDIT | Page 19 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 5.9. | Analysis and maturities of liabilities |
| Financial borrowings and debt (b) |
Gross amount |
Degree of liability repayment | ||||||||||||||
| Maturities of less than 1 year |
Maturities | |||||||||||||||
| Of more than 1 year |
Of more than 5 years |
|||||||||||||||
| Convertible debenture loans (1) |
9,484 | 9,484 | ||||||||||||||
| Other debenture loans (1) |
||||||||||||||||
| Loans and debt from credit institutions (1) |
||||||||||||||||
| – less than 1 year at inception |
||||||||||||||||
| – more than 1 year at inception |
360,000 | 360,000 | ||||||||||||||
| Other loans and financial debt (1)(2) |
2,700,662 | 2,700,662 | ||||||||||||||
| Trade accounts payable |
1,191,929 | 1,191,929 | ||||||||||||||
| Personnel and related expenses |
356,314 | 356,314 | ||||||||||||||
| Social security and related liabilities |
390,482 | 390,482 | ||||||||||||||
| Income tax |
||||||||||||||||
| Value added tax |
||||||||||||||||
| Secured bonds |
||||||||||||||||
| Other taxes and similar |
44,482 | 44,482 | ||||||||||||||
| Trade accounts payable – fixed assets |
||||||||||||||||
| Trade accounts payable to group companies and associates (2) |
||||||||||||||||
| Other payables (including payables on repurchase agreements) |
2,535 | 2,535 | ||||||||||||||
| Payables on borrowed securities |
||||||||||||||||
| Deferred income |
240,000 | 240,000 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| TOTAL |
5,295,888 | 5,295,888 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| (1) | Loans taken out during the year |
| (1) | Loans repaid during the year |
| (2) | Loans and debt contracted with individual associates |
Trade accounts payables consist mainly of €376,135 in invoices not yet received and €1,191,929 in supplier invoices.
| CPA AUDIT | Page 20 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 5.10. | Accrued expenses and credit notes to be issued |
(Article R.123-189 of the French Commercial Code)
| ACCRUED CHARGES INCLUDED IN THE FOLLOWING BALANCE SHEET ITEMS |
Fiscal year ended | Fiscal year ended | ||||||
| 12/31/2022 | 12/31/2021 | |||||||
| Convertible debenture loans |
||||||||
| Other debenture loans |
||||||||
| Loans and debt from credit institutions |
||||||||
| Other loans and financial debt |
||||||||
| Trade accounts payable |
376,135 | 498,760 | ||||||
| Debts Tax and payroll |
529,808 | 477,889 | ||||||
| Trade accounts payable – fixed assets |
||||||||
| Other debts |
||||||||
|
|
|
|
|
|||||
| TOTAL |
905,943 | 976,649 | ||||||
|
|
|
|
|
|||||
| 5.11. | Deferred income and expenses |
| DEFERRED INCOME |
Fiscal year ended | Fiscal year ended | ||||||
| 12/31/2022 | 12/31/2021 | |||||||
| Operating income |
240,000 | |||||||
| Financial income |
||||||||
| Extraordinary income |
||||||||
|
|
|
|||||||
| TOTAL |
240,000 | |||||||
|
|
|
|||||||
| PREPAID EXPENSES |
Fiscal year ended | Fiscal year ended | ||||||
| 12/31/2022 | 12/31/2021 | |||||||
| Operating expenses |
32,412 | 21,904 | ||||||
| Financial expenses |
||||||||
| Extraordinary expenses |
||||||||
|
|
|
|
|
|||||
| TOTAL |
32,412 | 21,904 | ||||||
|
|
|
|
|
|||||
| 5.12. | Capitalized production |
Capitalized production for the period amounts to €3,155,303 and corresponds to development costs capitalized during the period.
| CPA AUDIT | Page 21 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
| 5.13. | Other external expenses and purchases |
As of December 31, 2022, other external expenses and purchases are broken down as follows:
| Other external expenses and purchases |
12/31/2022 | 12/31/2021 | ||||||
| Sub-contracting |
2,356,723 | 1,045,683 | ||||||
| IT consulting |
7,765 | 23,315 | ||||||
| Electricity |
25,372 | 36,903 | ||||||
| Purchase of small equipment and consumables |
891,758 | 437,572 | ||||||
| Managed services |
6,912 | 47,158 | ||||||
| Equipment leasing |
100,566 | 58,217 | ||||||
| Rentals |
271,608 | 259,294 | ||||||
| Rental charges |
164,438 | 138,606 | ||||||
| Maintenance and repairs |
55,452 | 43,022 | ||||||
| Insurance |
43,846 | 31,438 | ||||||
| Documentation |
53,896 | 5,518 | ||||||
| Temporary staff |
3,500 | 0 | ||||||
| Legal costs and fees |
1,744,124 | 1,164,965 | ||||||
| Advertising, catalogs and printed materials |
1,275 | 3,000 | ||||||
| Transportation of goods |
116,414 | 36,987 | ||||||
| Travel and entertainment expenses |
75,257 | 26,433 | ||||||
| Telephone and internet |
17,813 | 10,339 | ||||||
| Bank services |
6,590 | 5,683 | ||||||
| Dues and other contributions |
17,654 | 12,141 | ||||||
|
|
|
|
|
|||||
| Total |
5,960,963 | 3,386,273 | ||||||
|
|
|
|
|
|||||
| 5.14. | Non-recurring income from operations |
Extraordinary income relates to the share of investment grants recognized in the income statement in the amount of €355,974 and to the debt write-off on the advance received from BPI France for the PHAGOSCLIN project in the amount of €240 thousand.
Grant income recognized in fiscal year 2022 amounted to €355 thousand: €102 thousand for PHAGOGRAM and €253 thousand for PHAGECOLI.
For the record:
| • | PHAGOGRAM grant received from BPI France |
| • | Total amount of the grant: euros253,763 |
| • | Funds received as of December 31, 2022: euros253,763 |
| • | PHAGECOLI grant received from BPI France |
| • | Total amount of the grant: €1,012,680 |
| • | Funds received as of December 31, 2022: euros253,170 |
| 5.15. | Corporate tax and loss carryforwards |
As the Company does not generate taxable income, it does not incur any tax expenses. The amount recognized in the income statement for corporate income tax is composed of the Research Tax Credit (RTC).
The Company’s research and development work generated an RTC of €989 thousand for 2021, for which reimbursement was requested and received in September 2022.
| CPA AUDIT | Page 22 |
PHERECYDES PHARMA Financial situation as of 12/31/2022
For 2022, the research tax credit is estimated at €1,388 thousand.
The calculation bases are as follows:
| Base |
June 22 | |||
| Personnel |
1,838,202 | |||
| Charges |
64,260 | |||
| Patents |
268,138 | |||
| R&D sub-contracting |
2,416,694 | |||
| Grant |
942,602 | |||
| 5.16. | Additional information |
| • | Average workforce |
| Paid staff | Staff seconded to the company | |||||||
| Employees |
27 | |||||||
|
|
|
|||||||
| TOTAL |
27 | |||||||
|
|
|
|||||||
| • | Off-balance sheet commitments |
Retirement bonuses
As of December 31, 2021, the estimated amount of the retirement bonus obligation was €100,366.
As of December 31, 2022, considering the company’s data and the actuarial assumptions used, mainly a gross discount rate of 0.98%, the total retirement bonus liability amounts to €67,403.
The following assumptions were used:
| • | Change in compensation: 1.5% |
| • | Turn-over: average |
| • | Mortality: INSEE 2019 |
| • | Retirement age: 62 years |
See note 4.10
Donations
Donations of batches of bacteriophage cocktails and placebo products with an estimated value of €380 thousand have been pledged on the condition that a future clinical trial agreement is entered into.
| • | Related parties |
There were no significant transactions with related parties during the year that were not concluded under normal market conditions.
| CPA AUDIT | Page 23 |
STATUTORY AUDITORS REPORT ON THE ANNUAL FINANCIAL STATEMENTS FOR THE FISCAL YEAR
ENDED DECEMBER 31, 2022
69
PHERECYDES PHARMA
Statutory Auditors report on the
annual financial statements
(Fiscal year ended December 31, 2022)

Statutory Auditors report on the annual
financial statements
(Fiscal year ended December 31, 2022)
To the General Meeting of Shareholders
PHERECYDES PHARMA
22, Boulevard Benoni Goullin
44200 Nantes, France
Opinion
In compliance with the assignment entrusted to us by your articles of association, we have audited the annual financial statements of the company PHERECYDES PHARMA for the fiscal year ended December 31, 2022, as attached to this report.
We hereby certify that the annual financial statements are consistent with French accounting rules and principles and give a true and fair view of the operating results for the fiscal year just ended and of the financial position and assets and liabilities of the company at the end of that fiscal year.
Basis of opinion
Audit framework
We carried out our audit in accordance with the professional standards applicable in France. We believe that the information we have obtained is sufficient and appropriate to provide a basis for our opinion.
Our responsibilities under these standards are outlined in the “Responsibilities of the Statutory Auditor in relation to the audit of the annual financial statements” section of this report.
Independence
We have carried out our audit in accordance with the rules of independence provided for in the French Commercial Code and in the Code of Ethics for Statutory Auditors for the period from January 1, 2022 to the issue date of our report.
Observations
Without calling into question the opinion expressed above, we would like to draw your attention to note 2 to the annual financial statements, which sets out the information underlying the maintenance of operating principle.
PricewaterhouseCoopers Audit, S.A.S., 63, rue de Villiers 92208 Neuilly-sur-Seine, France
Cedex Telephone: +33 (0)1 56 57 58 59, www.pwc.fr
Accounting firm registered with the Paris – Ile de France association of chartered accountants. Statutory auditing firm, member of the Versailles and Centre regional associations of statutory auditors. Simplified joint stock company (Société par Actions Simplifiée) with capital of €2,510,460. Registered office: 63 rue de Villiers 92200 Neuilly-sur-Seine, France. Nanterre Trade and Companies Register 672 006 483. VAT no. FR 76 672 006 483. Siret 672 006 483 00362. APE Code 6920 Z. Offices: Bordeaux, Grenoble, Lille, Lyon, Marseille, Metz, Nantes, Neuilly-Sur-Seine, Nice, Poitiers, Rennes, Rouen, Strasbourg, Toulouse.
PHERECYDES PHARMA
Statutory Auditors report on the annual financial statements
for the fiscal year ended December 31, 2022 – Page 2
Justification of assessments
Pursuant to the provisions of Articles L.823-9 and R.823-7 of the French Commercial Code relating to the justification of our assessments, we hereby inform you that the most significant assessments that we have made, in our professional opinion, concerned the appropriateness of the accounting principles applied:
| • | As part of our assessment of the accounting principles applied by your company, we examined the methods used to capitalize development costs and ensured that the notes to the financial statements entitled “Research and development costs,” “Statement of fixed assets,” “Fixed assets” and “Depreciation, amortization and impairment” provide appropriate information. |
These assessments were made in the context of our audit of the annual financial statements taken as a whole and the formation of our opinion expressed above. We do not express our opinion on any individual item in these annual financial statements.
Specific verifications
In accordance with professional standards applicable in France, we also performed the specific verifications required by law and regulations.
Information provided in the management report and in other documents on the financial situation and the annual financial statements sent to the shareholders
We have no observations to make with regard to the fair presentation of the information provided in the management report of the Board of Directors and in the other documents addressed to shareholders relating to the financial position and the annual financial statements, nor do we have any observations to make with regard to the conformity of such documents with the annual financial statements.
We hereby attest to the fair presentation of the information relating to the payment periods mentioned in Article D.441-6 of the French Commercial Code, and to its conformity with the annual financial statements.
Report on corporate governance
We hereby certify that the report of the Board of Directors on corporate governance contains the information required by Article L.225-37-4 of the French Commercial Code.
Other information
Pursuant to the law, we have ensured that other information relating to the identity of shareholders or voting right holders have been communicated in the management report.
Responsibilities of management and those charged with corporate governance in relation to the annual financial statements
It is the responsibility of management to prepare the annual financial statements in such a way that they give a true and fair view in accordance with French accounting rules and principles, and to implement any internal control procedures it deems necessary to ensure that the annual financial statements are free from material misstatement, whether due to fraud or error.
PHERECYDES PHARMA
Statutory Auditors report on the annual financial statements
for the fiscal year ended December 31, 2022 – Page 3
When preparing the annual financial statements, it is the responsibility of management to evaluate the company’s ability to continue as a going concern, to disclose in such financial statements, where appropriate, the necessary information on the going concern and to apply the going concern accounting policy, unless there are plans for the company to be liquidated or to cease its activities.
The annual financial statements have been approved by the Board of Directors.
Responsibilities of the Statutory Auditor in relation to the audit of the annual financial statements
It is our responsibility to prepare a report on the annual financial statements. Our objective is to obtain reasonable assurance that the annual financial statements as a whole are free of material misstatement. Reasonable assurance is a high level of assurance but does not guarantee that an audit carried out in accordance with professional standards will systematically detect any material misstatement. Misstatements may arise from fraud or error and are considered material when one can reasonably expect that they may, individually or together, influence the economic decisions that users of the financial statements make based on them.
As specified in Article L.823-10-1 of the French Commercial Code, our role in certifying the financial statements is not to guarantee the viability or quality of the management of your company.
As part of an audit carried out in accordance with the professional standards applicable in France, the Statutory Auditor exercises their professional judgment throughout the audit. In addition:
| • | they identify and assess the risks of material misstatement in the annual financial statements, whether due to fraud or error; define and implement audit procedures to address such risks; and collect information that they believe is sufficient and appropriate to provide a basis for their opinion. The risk of not detecting material misstatement resulting from fraud is higher than the risk of not detecting material misstatement resulting from error, as fraud may involve collusion, falsification, deliberate omissions, misrepresentation or the circumvention of internal control; |
| • | they become aware of the internal control relevant to the audit in order to define audit procedures that are appropriate to the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the internal control; |
| • | they assess the appropriateness of the accounting methods used and the reasonableness of the accounting estimates made by management, as well as the related information provided in the annual financial statements; |
| • | they assess the appropriateness of management’s application of the going concern accounting policy and, based on the information collected, whether there is any material uncertainty related to events or circumstances likely to affect the company’s ability to continue as a going concern. This assessment is based on the information collected up to the date of the report, bearing in mind that circumstances or subsequent events may call into question the company’s ability to continue as a going concern. If they conclude that a material uncertainty exists, they draw the attention of the readers of their report to the information provided in the annual financial statements concerning this uncertainty or, if this information is not provided or is not relevant, they issue a qualified opinion or refuse to certify; |
PHERECYDES PHARMA
Statutory Auditors report on the annual financial statements
for the fiscal year ended December 31, 2022 – Page 4
| • | they assess the overall presentation of the annual financial statements and whether the annual financial statements reflect the underlying transactions and events in such a way that gives a true and fair view. |
Signed in Neuilly-sur-Seine, France, April 26, 2023
Statutory Auditor
PricewaterhouseCoopers Audit
Cédric Mazille
STATUTORY AUDITORS SPECIAL REPORT ON RELATED-PARTY AGREEMENTS
71
PHERECYDES PHARMA
Statutory Auditors special report on related-party agreements
(General Meeting of Shareholders to approve
the financial statements for the fiscal year ended
December 31, 2022)

Statutory Auditors special report on related- party agreements
(General Meeting of Shareholders to approve the financial statements for the fiscal year ended December 31, 2022)
To the General Meeting of Shareholders of the company,
PHERECYDES PHARMA
22, Boulevard Benoni Goullin
44200 Nantes, France
In our capacity as Statutory Auditors of your company, we hereby present our report on related-party agreements.
Our responsibility is to inform you, based on the information provided to us, of the characteristics, the essential terms and conditions and the reasons justifying the company’s interest in the agreements of which we have been informed or of which we may have become aware in the course of our assignment, without commenting on their usefulness or appropriateness or on the existence of other agreements. It is your responsibility, under the terms of Article R.225-58 of the French Commercial Code, to assess the benefits resulting from entering into these agreements prior to their approval.
In addition, it is our responsibility, where applicable, to provide you with the information required by Article R.225-58 of the French Commercial Code relating to the execution, during the past fiscal year, of agreements already approved by the General Meeting of Shareholders.
We have performed the procedures we’ve deemed necessary to comply with the professional standards issued by the French Institute of Statutory Auditors relating to this assignment.
AGREEMENTS SUBMITTED TO THE APPROVAL OF THE GENERAL MEETING OF SHAREHOLDERS
We hereby inform you that we have not been advised of any agreements authorized and entered into during the past fiscal year that should be submitted to the approval of the General Meeting of Shareholders pursuant to the provisions of Article L.225-86 of the French Commercial Code.
PricewaterhouseCoopers Audit, S.A.S., 63, rue de Villiers 92208 Neuilly-sur-Seine, France Cedex Telephone: +33 (0)1 56 57 58 59, www.pwc.fr
Accounting firm registered with the Paris – Ile de France association of chartered accountants. Statutory auditing firm, member of the Versailles and Centre regional associations of statutory auditors. Simplified joint stock company (Société par Actions Simplifiée) with capital of €2,510,460. Registered office: 63 rue de Villiers 92200 Neuilly-sur-Seine, France. Nanterre Trade and Companies Register 672 006 483. VAT no. FR 76 672 006 483. Siret 672 006 483 00362. APE Code 6920 Z. Offices: Bordeaux, Grenoble, Lille, Lyon, Marseille, Metz, Nantes, Neuilly-Sur-Seine, Nice, Poitiers, Rennes, Rouen, Strasbourg, Toulouse.
PHERECYDES PHARMA
Statutory Auditors special report on related-party agreements
(General Meeting of Shareholders to approve the financial statements for the fiscal year ended December 31, 2022) – Page 2
AGREEMENTS ALREADY APPROVED BY THE GENERAL MEETING OF SHAREHOLDERS
We hereby inform you that we have not been advised of any agreement previously approved by the General Meeting of Shareholders whose execution has continued during the past fiscal year.
Signed in Neuilly-sur-Seine, France, April 26, 2023
Statutory Auditor
PricewaterhouseCoopers Audit

Cédric Mazille


